Need for prospective collection of experience and repeated samples in esophageal squamous cell carcinoma
I appreciate the comments by Ajani, Pectasides, and RJ Kelly on our recent report (1). To my knowledge, two studies have evaluated checkpoint inhibitors related to esophageal squamous cell carcinoma (ESCC) (1,2). These two studies reported similar results with an objective response rate of approximately 20%, although selection bias should be considered. Several factors are thought to have influenced these results. As suggested by Ajani et al., previous treatment with radiotherapy resulted in longer progression-free survival and overall survival with immune checkpoint inhibitors (ICIs); these effects were not simply because of the combination of ICIs and radiotherapy. Radiotherapy has been recently identified as a relevant factor for immune oncology (3). Therefore, an analysis of its tentative anti-tumor effect, as well as the whole oncological course, is needed. Conversely, surgery—especially if there are complications such as infection—could have a negative impact on immuno-sensitivity. Esophagectomy for esophageal cancer is one of the most invasive operative procedures. Surgical stress may induce the release of pro-inflammatory cytokines, and cytokine overproduction can result in systemic inflammatory response syndrome, which may lead to acute lung injury and multiple organ dysfunction syndrome. Such surgical stress may cause immuno-suppression, affecting perioperative mortality, survival, and immune response (4).
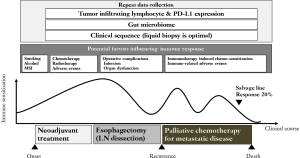
Post-immunotherapy-induced hyper-chemosensitization has been recently investigated because post-immunotherapy patients show a favorable overall response (5). The immuno-modulatory effects of chemotherapy appear to improve survival when administered prior to chemotherapy. A report on the association of immune-related adverse events (irAEs) with ICIs (6) showed that development of irAEs was associated with better survival. An early onset, irAEs might be predictive of and maximize the therapeutic effect of these agents, although the mechanisms are unknown. Long-lasting shrinkage of tumor masses after the discontinuation of ICIs is another unique phenomenon to be considered as a treatment strategy (7). This immunotherapy-specific phenomenon should be considered for each disease-specific strategy. The results of ICIs for ESCC (overall response rate: 20%) may seem minimal. Anti-tumor effects may be maximized if we set up ICIs at the best sequence; however, we have no definitive data on the best point for immuno-treatment for ESCC (Figure 1).
As several recent investigations of immuno-therapy have reported unexpected results, it could be said that, in the immuno-oncology era, classical oncological indicators are not suitable for the early detection for these new strategies (8). Early investigation by objective response is insufficient for surrogacy for identifying promising treatments for immune-oncology. Rather, we need to define the most appropriate primary endpoint of ICIs (9).
It could also be said that traditional oncological investigational strategies cannot improve survival. A simple combination of chemotherapy, molecular target agents, and/or radiation is typically used to improve survival. However, these approaches do not lead to immunological specific benefits. Pseudo-progression, hyper-progression, and immunotherapy-induced chemo-sensitization are the typical unexpected and unevaluated results, and these unique new phenomena cannot be evaluated by the standard oncological approach. For evaluation, we need more experience and large cohort data with a variety of samples. In the near future, it may be necessary to redefine the overall strategy and treatment sequence for ESCC in favor of multi-modal treatments.
There are still two important issues that require discussion. One is how to interpret clinical sequence data, which is essential for advancing treatment for ESCC. The other important issue is how to utilize data from fecal samples, as the gut microbiome is known to influence the efficacy of immunotherapy (10). Fecal microbiota transplantation (FMT) from cancer patients who responded to ICIs is one of the most promising immunization strategies. As there are few studies on the role of the microbiota in ESCC, sample collection from prospective cohort trials is needed to gather information that can be used to inform treatment strategies.
In conclusion, there are several suggestions for improving survival in ESCC patients. To overcome ESCC by ICIs, a specific strategy is needed that incorporates experience, clinical sequence, and microbiota data. Prospective cohort trials with repeated collection of biopsy samples, liquids, and feces are thus warranted.
Acknowledgements
I thank Kenta Kawasaki and Akiyoshi Kasuga for their kind suggestion. Research grant from Ono pharm. Bristol-Meyers Squibb, MSD. An advisory board member for BMS and Ono pharma.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Kudo T, Hamamoto Y, Kato K, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol 2017;18:631-9. [Crossref] [PubMed]
- Doi T, Piha-Paul SA, Jalal SI, et al. Safety and Antitumor Activity of the Anti-Programmed Death-1 Antibody Pembrolizumab in Patients With Advanced Esophageal Carcinoma. J Clin Oncol 2018;36:61-7. [Crossref] [PubMed]
- Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895-903. [Crossref] [PubMed]
- Okamura A, Takeuchi H, Matsuda S, et al. Factors affecting cytokine change after esophagectomy for esophageal cancer. Ann Surg Oncol 2015;22:3130-5. [Crossref] [PubMed]
- Schvartsman G, Peng SA, Bis G, et al. Response rates to single-agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non-small cell lung cancer. Lung Cancer 2017;112:90-5. [Crossref] [PubMed]
- Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 2018;4:374-8. [Crossref] [PubMed]
- Kimura H, Sone T, Murata A, et al. Long-lasting shrinkage in tumor mass after discontinuation of nivolumab treatment. Lung Cancer 2017;108:7-8. [Crossref] [PubMed]
- Hodi FS, Ballinger M, Lyons B, et al. Immune-Modified Response Evaluation criteria In Solid Tumors (imRECST): Refining Guidelines to Assess the Clinical Benefit of Cancer Immunotherapy. J Clin Oncol 2018;36:850-8. [Crossref] [PubMed]
- Ritchie G, Gasper H, Man J, et al. Defining the Most Appropriate Primary End Point in Phase 2 Trials of Immune Checkpoint Inhibitors for Advanced Solid Cancers: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:522-8. [Crossref] [PubMed]
- Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018;359:91-7. [Crossref] [PubMed]