Lung function in the late postoperative phase and influencing factors in patients undergoing pulmonary lobectomy
Introduction
Pulmonary lobectomy is a common surgical intervention with consensus support as a standard procedure for curable treatment of primary non-small cell lung cancer (NSCLC) (1). However, resection of lung parenchyma impairs some reserves of lung function, possibly leading to inability to tolerate exercise because alveolar units cannot be reproduced to fully compensate for lost lung volume. Vital capacity (VC) and forced expiratory volume in 1 second (FEV1) are representative indexes of lung function, and are routinely measured before surgery to select suitable candidates for pulmonary lobectomy. Therefore, surgeons are required to predict loss of lung volume quantified by these measures, and also subsequent recovery caused by morphological expansion of the remaining lung.
Previous studies have assessed lung function after pulmonary lobectomy, and have determined that VC and FEV1 decrease for a period of approximately 6 months after surgery (2-4). Other investigations have reported that these values gradually recover after 6 months and then are maintained 12 months after surgery (5). However, these reports included a relatively small sample sizes. Moreover, details of lung function >1 year after surgery remain uncertain.
The aim of this study was to compare pre- and postoperative values of VC and FEV1 in the late postoperative phase (>1 year after surgery) in a comparatively larger sample size of patients who underwent pulmonary lobectomy. In addition, patient characteristics, including comorbidities that potentially affect lung function after lobectomy, were also investigated.
Methods
Patients
The present study included patients who underwent pulmonary lobectomy through thoracotomy with antero-axillar incision primarily for NSCLC or other pulmonary diseases at Kurume University Hospital (Kurume, Japan) or Oita Prefecture Saiseikai Hita Hospital (Hita, Japan) between March 2000 and June 2014. Patients older than 80 years of age, with poor physical activity (Eastern Cooperative Oncology Group performance status equal to or worse than 2), and those who received perioperative chemo- or radiotherapy, except postoperative oral uracil and tegafur, were excluded because of the potential for inadequate performance in spirometry test due to physical dysfunction (6). In addition, patients who were lost to follow-up were excluded; ultimately, 112 patients were enrolled in the present analysis. Histological subtypes of NSCLC were assigned according to the classification of the World Health Organization (7). The study was approved by the Institutional Research Ethics Committee of Kurume University (No. 17065) and Oita Prefecture Saiseikai Hita Hospital (No. 26-11), and all patients provided informed written consent to participate.
Assessment of pulmonary function
Lung function was evaluated using spirometry according to American Thoracic Society/European Respiratory Society criteria (8). To calculate predicted postoperative VC and FEV1, the lungs were divided into a total of 42 subsegments. The subsegments were then assessed for obstruction using computed tomography imaging or bronchoscopy, and predicted postoperative VC and FEV1 were calculated based on the number of functioning/unobstructed subsegments that would be removed during surgery (9,10). The predicted postoperative value of VC and FEV1 were calculated using the following equation:
Preoperative value × [1 − (b − n)/(42 − n)] (L),
In which n and b are the numbers of obstructed segments and total segments, respectively (5). In addition, decreasing rate, which was defined as the decrease in the proportion of observed postoperative lung volume compared with preoperative lung volume, was similarly calculated as follows:
[(preoperative value – postoperative value)/preoperative value] ×100 (%).
Patient characteristics potentially effecting a decrease in pulmonary function were also analyzed and included sex, smoking habits, resected lobe, tumor histological subtype, interval between pre- and postoperative measurement, and comorbidities. In the present study, the presence of chronic obstructive pulmonary disease (COPD) as a comorbidity was defined according to the Global Initiative for Obstructive Lung Disease (GOLD) criteria (11).
Statistical analysis
Statistical comparisons between the resected lobe and clinicopathological features were evaluated using Wilcoxon rank sum tests, paired t-tests, and Fisher’s exact tests. The paired t-test was performed to compare preoperative and postoperative lung volume, as well as predicted and observed decreasing rates of VC and FEV1. Simple regression and stepwise multiple regression models were used in univariate and multivariate analyses to examine the influence of each characteristic in the observed decreasing rate in VC and FEV1; P<0.05 was considered to be statistically significant. Statistical analyses were all performed using JMP Pro version 11 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
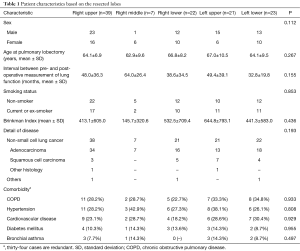
Table 1 summarizes the characteristics of the 112 patients enrolled in the present study according to the lobe that was resected. Subjects included 64 males and 48 females, with a mean age of 65.1 and 68.9 years at pre- and postoperative measurement of lung function, respectively. There were 61 (54.5%) non-smokers and 51 (45.5%) patients who had ever smoked, with a mean Brinkman Index of 469, which is defined as (number of cigarettes per day) × (number of years for which a person smoked) (12). Comorbidities included COPD in 33 (29.5%) patients, hypertension in 34 (30.4%), cardiovascular disease in 28 (25.0%), diabetes mellitus in 13 (11.6%), and bronchial asthma in 9 (8.0%). There were no statistical differences in clinicopathological features of patients with regard to the resected lobe.
Full table
Comparison between pre- and postoperative lung function after pulmonary lobectomy
The mean interval from pre- to postoperative measurement of lung function was 44.3 months (range, 12–186 months). Calculated postoperative decreases in VC were 10.5%±1.8% in patients who underwent right upper lobectomy (RU), 7.2%±1.5% for right middle lobectomy (RM), 14.3%±2.3% for right lower lobectomy (RL), 16.6%±3.0% for left upper lobectomy (LU), and 14.7%±2.5% for left lower lobectomy (LL). These postoperative decreases were all statistically significant, regardless of the lobe that was resected (Table 2). Similarly, calculated postoperative decreases in FEV1 were 14.8%±1.8% in patients who underwent RU, 11.9%±4.0% for RM, 14.9%±2.3% for RL, 17.9%±2.9% for LU, and 15.1%±2.4% for LL; patients underwent all procedures significantly suffered a notable loss of FEV1 after pulmonary lobectomy as observed in VC (Table 2).

Full table
Discrepancy between predicted and observed lung function after pulmonary lobectomy
The actual decreasing rate of VC in the late postoperative phase was overestimated in patients who underwent RU, RL, LU, and LL (P=0.044, P<0.001, P=0.028, and P=0.003, respectively) (Table 3). In contrast, a substantial decreasing rate in FEV1 was overestimated only in patients who underwent RL and LL, differences that were statistically significant (P<0.001 and P=0.006, respectively) (Table 3).

Full table
Univariate and multivariate analysis of factors affecting decreasing volume of VC and FEV1
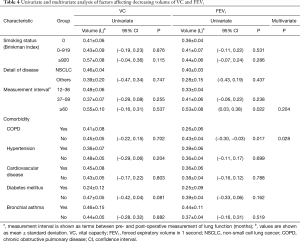
Selected patient characteristics were assessed to determine whether any influenced the decreasing volume of VC and FEV1 (Table 4). In the analysis of VC, none of the variables affected the decreasing volume in univariate analysis, which precluded their inclusion in further multivariate analyses. In FEV1, the interval between pre- and postoperative measurement of lung function, and the presence of COPD, were determined to be significant factors affecting lung volume loss (P=0.022 and P=0.017, respectively). In the multivariate analysis, only the presence of COPD was identified as an independent factor influencing lung volume loss (P=0.028).
Full table
Discussion
In the present study, we demonstrated that VC and FEV1 clearly decreased after pulmonary lobectomy in the late postoperative phase (>1 year after surgery). However, postoperative lung function in terms VC was better preserved than predicted in patients who underwent RU, RL, LU, and LL. In addition, patients who underwent RL and LL exhibited significantly better preservation of FEV1 than predicted compared with those who underwent other procedures. The presence of COPD was identified as an independent predictor of better preservation of FEV1; specific profiles possibly affecting VC, however, were not determined. These results are useful, and can be efficaciously used in the management of therapeutic strategies for NSCLC, particularly in decisions regarding surgical procedures.
Predicted postoperative FEV1 evaluated using spirometry is one of the measurements commonly applied to the prediction of postoperative mortality and morbidity after lung resection; therefore, it is widely used to select suitable candidates for pulmonary lobectomy (10,13). Several approaches have similarly been adopted in recent years to predict residual lung function after pulmonary lobectomy, such as bronchial arteriography, pulmonary arteriography, pulmonary blood flow scintigraphy, and computed tomography-based lung volumetry (13-16). In contrast to these modalities, spirometry testing can be performed easily because of its long-standing and widespread availability (9). To further advance the understanding of processes that occur in the remaining lung after pulmonary lobectomy, additional studies investigating the correlation between results measured using spirometry testing and other modalities are required.
Our study clarified that pulmonary lobectomy necessarily entail significant decrease in VC and FEV1 after the procedure, even in the late phase >1 year after surgery compared with preoperative values. Previous studies have determined that lung function decreases in the early postoperative period after pulmonary lobectomy (17,18), gradually recovers after 6 months, and then maintain 12 months after surgery (2-5,19). Acknowledging these findings, our result serves as confirmation that, even after maximal recovery of lung function―reflected by compensation of the remaining lung―lung function after pulmonary lobectomy declines significantly compared with preoperative values. Furthermore, our results also showed that the decreasing volume in the late postoperative phase was larger when the interval between pre- and postoperative measurement of lung function was longer (Table 4), which is consistent with previous reports that maximal recovery after pulmonary lobectomy reached a peak approximately 1 year after surgery. Because of major concerns regarding poor respiratory function elicited by lung resection, which leads to postoperative long-term disability and poor quality of life, these results should be clearly reflected in patient selection for pulmonary lobectomy in clinical settings.
Patients who underwent RL and LL exhibited significantly better preservation of both VC and FEV1 than predicted >1 year after pulmonary lobectomy. Similarly, Ueda et al. reported that patients who underwent lower lobectomy exhibited significantly better lung function recovery than those who underwent upper lobectomy at 6–12 months after surgery, even though lower lobectomy required a volumetrically larger resection than upper lobectomy based on computed tomography-based functional lung volumetry and spirometry tests (20). They speculated that this result could be attributed to a compensatory response after lower lobectomy, which appeared to be more robust rather than that after upper lobectomy. Nevertheless, explanation for these results may be supported by some studies reporting that the compensatory inflation of the remaining lung after lobectomy was accompanied by increased pulmonary ventilation and perfusion of the remaining lung (21). Another study, involving adult dogs that included microscopic and radiologic evaluation, also demonstrated that compensatory expansion of the remaining lung is not simply a consequence of hyperinflation of the pre-existing alveolar septal tissue, but is accompanied by some increase in the vital lung tissue (3,22). Although further studies are needed to identify detailed mechanisms of this outcome, it is highly likely that lung function recovery of FEV1 in patients who undergo RL or LL could be expected to be better preserved than that in those who undergo other procedures.
Univariate and multivariate analysis indicated that comorbid COPD was an independent favorable factor for the preservation of FEV1 in the late postoperative phase. Similarly, Baldi et al. reported that patients with mild to severe COPD exhibited better preservation of lung function in the late phase after pulmonary lobectomy than healthy patients (23). To investigate the basis of this result, we performed an additional analysis and demonstrated that the observed/predicted ratios of FEV1 were higher in COPD patients compared with non-COPD patients. This finding is consistent with previous studies reporting minimal loss―or even improvement―of pulmonary function after lobar resection in COPD patients. On the other hand, other authors have speculated that a ventilation-perfusion relationship may be associated with this outcome (24,25) and, therefore, patients with comorbid COPD can expect better preservation of FEV1 after pulmonary lobectomy in the late postoperative phase.
There were several limitations in this study. First, although we defined the lung function after lobectomy in the late postoperative phase as those >1 year following surgery, the interval between pre- and postoperative measurement of lung function varied among patients. This was caused by operational inconsistencies in our clinical follow-up, in which spirometry tests after surgery are not routinely performed. Further studies, including measurements of lung function in a synchronized, rigorously defined, period in the late phase following pulmonary lobectomy, are necessary to confirm our results because statistical biases may have originated from factors such as aging and the natural progression of underlying COPD. Second, this study did not analyze consecutive patients who underwent pulmonary lobectomy because some were lost to follow-up. To avoid a statistical bias due to patient selection, a prospective case series is necessary in future studies.
Conclusions
Our results demonstrated that lung function after pulmonary lobectomy inevitably decreased compared with the preoperative measurements, even after the recovery of lung function reached a peak after surgery. Furthermore, better preservation of respiratory function in the late postoperative phase can be expected in patients scheduled for RL and LL, as well as those with comorbid COPD.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Research Ethics Committee of Kurume University (No. 17065) and Oita Prefecture Saiseikai Hita Hospital (No. 26-11), and all patients provided informed written consent to participate.
References
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Nezu K, Kushibe K, Tojo T, et al. Recovery and limitation of exercise capacity after lung resection for lung cancer. Chest 1998;113:1511-6. [Crossref] [PubMed]
- Ali MK, Ewer MS, Atallah MR, et al. Regional and overall pulmonary function changes in lung cancer. Correlations with tumor stage, extent of pulmonary resection, and patient survival. J Thorac Cardiovasc Surg 1983;86:1-8. [PubMed]
- Bolliger CT, Jordan P, Soler M, et al. Pulmonary function and exercise capacity after lung resection. Eur Respir J 1996;9:415-21. [Crossref] [PubMed]
- Funakoshi Y, Takeda S, Sawabata N, et al. Long-term pulmonary function after lobectomy for primary lung cancer. Asian Cardiovasc Thorac Ann 2005;13:311-5. [Crossref] [PubMed]
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. editors. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. Lyon, France: IARC, 2015.
- American Thoracic Society. European Respiratory Society. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med 2002;166:518-624. [Crossref] [PubMed]
- Nakahara K, Monden Y, Ohno K, et al. A method for predicting postoperative lung function and its relation to postoperative complications in patients with lung cancer. Ann Thorac Surg 1985;39:260-5. [Crossref] [PubMed]
- British Thoracic Society and Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party. BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax 2001;56:89-108. [Crossref] [PubMed]
- Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. [Crossref] [PubMed]
- Brinkman GL, Coates EO Jr. The effect of bronchitis, smoking, and occupation on ventilation. Am Rev Respir Dis 1963;87:684-93. [PubMed]
- Kristersson S, Lindell SE, Svanberg L. Prediction of pulmonary function loss due to pneumonectomy using 133 Xe-radiospirometry. Chest 1972;62:694-8. [Crossref] [PubMed]
- Kristersson S, Arborelius M Jr, Jungquist G, et al. Prediction of ventilatory capacity after lobectomy. Scand J Respir Dis 1973;54:315-25. [PubMed]
- Arborelius M Jr, Kristersson S, Lindell SE, et al. 133 Xe-radiospirometry and extension of lung cancer. Scand J Respir Dis 1971;52:145-52. [PubMed]
- Sengul AT, Sahin B, Celenk C, et al. Postoperative lung volume change depending on the resected lobe. Thorac Cardiovasc Surg 2013;61:131-7. [Crossref] [PubMed]
- Varela G, Brunelli A, Rocco G, et al. Predicted versus observed FEV1 in the immediate postoperative period after pulmonary lobectomy. Eur J Cardiothorac Surg 2006;30:644-8. [Crossref] [PubMed]
- Brunelli A, Refai M, Salati M, et al. Predicted versus observed FEV1 and DLCO after major lung resection: a prospective evaluation at different postoperative periods. Ann Thorac Surg 2007;83:1134-9. [Crossref] [PubMed]
- Mariano-Goulart D, Barbotte E, Basurko C, et al. Accuracy and precision of perfusion lung scintigraphy versus 133Xe-radiospirometry for preoperative pulmonary functional assessment of patients with lung cancer. Eur J Nucl Med Mol Imaging 2006;33:1048-54. [Crossref] [PubMed]
- Ueda K, Tanaka T, Hayashi M, et al. Compensation of pulmonary function after upper lobectomy versus lower lobectomy. J Thorac Cardiovasc Surg 2011;142:762-7. [Crossref] [PubMed]
- Ali MK, Mountain CF, Ewer MS, et al. Predicting loss of pulmonary function after pulmonary resection for bronchogenic carcinoma. Chest 1980;77:337-42. [Crossref] [PubMed]
- Ravikumar P, Yilmaz C, Dane DM, et al. Regional lung growth following pneumonectomy assessed by computed tomography. J Appl Physiol (1985) 2004;97:1567-74; discussion 1549. [Crossref] [PubMed]
- Baldi S, Ruffini E, Harari S, et al. Does lobectomy for lung cancer in patients with chronic obstructive pulmonary disease affect lung function? A multicenter national study. J Thorac Cardiovasc Surg 2005;130:1616-22. [Crossref] [PubMed]
- Edwards JG, Duthie DJ, Waller DA. Lobar volume reduction surgery: a method of increasing the lung cancer resection rate in patients with emphysema. Thorax 2001;56:791-5. [Crossref] [PubMed]
- Sekine Y, Iwata T, Chiyo M, et al. Minimal alteration of pulmonary function after lobectomy in lung cancer patients with chronic obstructive pulmonary disease. Ann Thorac Surg 2003;76:356-61. [Crossref] [PubMed]


