Robotic-assisted mediastinal surgery: the first Chinese series of 167 consecutive cases
Introduction
In June 2000, the approval of the Da Vinci surgical system by the American FDA marked the birth of the first commercial surgical robot being applied in an operating room. There has been a surge in the number of robotic thoracic surgeries since the first report of robotic lobectomy in 2002 by Melfi et al. (1). In 2016, mainland China completed 2,119 robotic thoracic operations, and completed a total of 4,737 cases over the years, including 1,180 mediastinal mass resections. There have been many reports on the practice of robotic-assisted mediastinal surgery, which suggests that the robotic system seems ideally suited for mediastinal lesions and has satisfactory post-operative outcomes (2-5). Since our first robotic mediastinal mass resection in 2009 to June 2017, our center has performed 167 robotic mediastinal surgeries. Here, we would like to report the results of our 167 cases; this study is currently the largest retrospective single-center study in mainland China. We would also like to share our experience with the use of the da Vinci surgical system for mediastinal mass resection.
Methods
We respectively reviewed all patients who underwent mediastinal surgery performed by one surgeon (Qingquan Luo) with the da Vinci surgical system from May 2009 to June 2017 at Shanghai Lung Tumor Clinical Medical Center, Shanghai Chest Hospital, Shanghai Jiao Tong University. The perioperative outcomes and long-term follow-up are analyzed in this study. The learning curve is also characterized in this study. This study was approved by the ethics board of Shanghai Chest Hospital (KS(P)1805).
Surgical technique
All procedures were performed by the same surgeon using a three-arm robotic technique. One assistant at the bedside places the trocars connects them with the robotic arms, and then enlarges the incision to remove the specimen at the end of surgery. All patients underwent satisfactory general anesthesia with double-lumen tracheal intubation. CO2 was inflated through the camera port with 6–8 mmHg pressure. All the specimens were taken extracorporeally using either a specimen bag or surgical glove.
Anterior mediastinal lesion
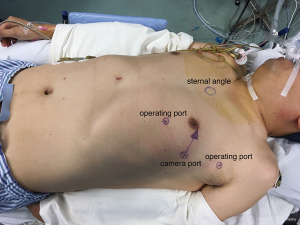
Patients with an anterior mediastinal mass are positioned with the affected side up at a 25- to 30-degree angle. The camera port is usually at the fifth intercostal space on the anterior-axillary line. The two robotic arm ports should be symmetrical with a line that connects the camera port and the tumor. Usually, one arm is at the third intercostal space close to mid-axillary line, and the other arm is at the fifth intercostal space at the mid-clavicular line. The camera port and robotic arms should be in a section of arc, adjusted to the patient’s figure (Figure 1). The right arm carries a permanent cautery hook that has an electrocautery function to perform resection. The left arm has Cadiere forceps (EndoWrist; Intuitive Surgical) to grasp the lesion. When the lesion is resected, the assistant then enlarges the robotic arm port incision to remove the specimen. The video shows robotic-assisted anterior mediastinal tumor resection (Figure 2).

Posterior mediastinal lesion
Patients with a posterior mediastinal lesion are placed with a contralateral decubitus position. The camera port is usually placed at the seventh intercostal space on the posterior axillary line. The robotic arm ports are usually at the sixth intercostal space on the anterior-axillary line and at the eighth intercostal space on the infrascapular line, with a distance of 7–8 cm from the camera port. Normally, we make an assistant incision in the fourth intercostal space on the anterior-axillary line to help expose the surgical field and remove the specimen.
Follow-up
The post-operative complications occurring within 30 days of surgery are evaluated according to the Clavien-Dindo classification (7). During the first year after surgery, the follow-up includes an enhanced chest CT scan every three months, every 6 months in the second year, and then once a year. For those patients with malignant tumors, we also recommend adjuvant chemotherapy or radiotherapy.
Results
General data
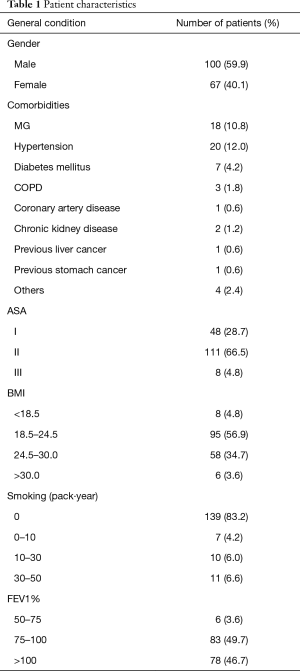
There were 167 patients, namely, 67 females and 100 males, with a mean age of 50.2 years (range, 12–78 years). The general data for the patients are shown in Table 1. The mean in-hospital cost was ¥64,563±¥10,899.67.
Full table
Surgical data and perioperative data
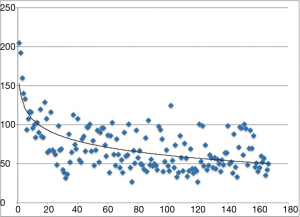
Complete resections were achieved in 164 cases, except for one case of squamous cell cancer of the thymus and two cases of angioma. The mean operation time and docking time are 70.01±29.49 and 10.12±2.77 min, respectively, with a mean blood loss of 60.34±115.38 mL. We analyzed the times of the first twenty, second twenty and last twenty mediastinal lesion resections as well as the docking time, as shown in Table 2. A significant difference in the operation time was observed between the first twenty operations and the last twenty operations. However, the operation times for the second twenty operations presented no significant difference with those of the last twenty operations. The learning curve is presented in Figure 3. Three cases (1.8%) were converted to sternotomy because of massive blood loss (>600 mL) in two cases and because of severe pleural adhesion in one case. The mean drainage on the first day after operation was 122.83±107.58 mL, and the mean drainage duration was 2.95 days, with a range of 1–7 days.

Full table
There was no intra- or perioperative death in these 167 patients. Grade II complications (according to the Clavien-Dindo classification) occurred in 5 (3%) patients. Two patients had atrial fibrillation and needed medical intervention. Three patients had high fever (>38.5 °C) after being discharged from the hospital and needed pharmacologic treatment.
Pathologic data
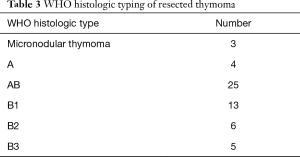
There were 56 thymomas, 52 cysts, 17 schwannomas, 9 bronchogenic cysts, 6 thymic hyperplasias, 6 foregut cysts, 4 squamous carcinomas, 3 teratomas, 2 neurofibromas, 2 angiomas, 2 epidermoid cysts, 2 thymic carcinoids, 1 pericardial cyst, 1 tracheoesophageal cyst, 1 ganglioneuroma, 1 sarcoidosis, 1 giant lymph node hyperplasia and 1 calcified pleural nodule. The average diameter of the mediastinal lesions was 3.85 cm, with a range of 0.40 to 10.00 cm. In total, 56 thymomas were classified according to the WHO and staged according to Masaoka, as presented in Tables 3,4.
Full table

Full table
Follow-up
The follow-up period ranged from 2–99 months, with a median follow-up of 28 months. MG symptoms were improved in all 18 (100%) patients who complained about myasthenia before the surgery. Adjuvant chemo/radiotherapy was recommended for patients diagnosed with Masaoka stages II and III. Among the 56 patients who underwent robotic thymectomies, five patients presented radiological evidence of recurrence: three patients underwent a second operation, while the other two had distant metastasis. One patient died due to progression of squamous cell cancer of the thymus.
Discussion
The robotic surgical system has greatly progressed in China in recent years, and it has unique advantages in posterior mediastinal lesion resection compared to open surgery and VATS (8). The robotic surgical system can provide a high-definition three-dimensional vision, interactive robotic arms with an EndoWrist system, a tremor filter and a conventional monitor console. Mediastinal lesions (including thymoma, thymic carcinoma, cyst, teratoma, and neurogenic tumor), especially anterior mediastinal mass, is the best indication for robotic surgery (9-11). There have been several studies comparing robotic mediastinal lesion resection to sternotomy and to VATS. Robotic mediastinal operation allows complete resection of mediastinal lesion and can reduce the incidence of post-operative complications and blood loss during the surgery with satisfactory long-term outcomes.
These 167 consecutive cases for a group of heterogeneous patients represent the largest experience of robotic mediastinal lesion resection in China and produced perioperative and long-term outcomes consistent with those of other recent reports (2,3,5,12). From our experiences, the position of the ports is of great importance to ensure complete resection of the lesion and access to the entire mediastinal space, especially for an anterior mediastinal mass. In anterior mediastinal lesion resection, the camera port should point to the mass at an angle of 45° to the anterior midline. The enhanced chest CT scan should be assessed before surgery by 1–2 experienced thoracic surgeons to evaluate the lesion and surrounding tissue. After 20 procedures, a surgeon can obtain a basic understanding of robotic-assisted mediastinal surgery.
At present, patients must pay extra money to undergo robotic surgery, which is not covered by medical insurance. This may explain the lower popularity of robotic surgery in China than in America.
Previous studies and our retrospective study have demonstrated that robotic-assisted mediastinal lesion surgery is safe and effective. Further prospective RCT studies are needed to verify the effectiveness and safety of robotic surgical systems.
Acknowledgements
Funding: This work was supported by Shanghai Hospital Development Center (Grant Number: SHDC12016113).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the ethics board of Shanghai Chest Hospital (KS(P)1805).
References
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864. [Crossref] [PubMed]
- Rea F, Schiavon M, Di CF, et al. Single-institution experience on robot-assisted thoracoscopic operations for mediastinal diseases. Innovations 2011;6:316-22. [PubMed]
- Melfi F, Fanucchi O, Davini F, et al. Ten-year experience of mediastinal robotic surgery in a single referral centre. Eur J Cardiothorac Surg 2012;41:847-51. [Crossref] [PubMed]
- Weksler B, Tavares J, Newhook TE, et al. Robot-assisted thymectomy is superior to transsternal thymectomy. Surg Endosc 2012;26:261-6. [Crossref] [PubMed]
- Seong YW, Kang CH, Choi JW, et al. Early clinical outcomes of robot-assisted surgery for anterior mediastinal mass: its superiority over a conventional sternotomy approach evaluated by propensity score matching. Eur J Cardiothorac Surg 2014;45:e68-73; discussion e73.
- Li H, Li J, Huang J, et al. Robotic-assisted anterior mediastinal tumor resection. Asvide 2018;5:522. Available online: http://www.asvide.com/article/view/24995
- Seely AJE, Ivanovic J, Threader J, et al. Systematic Classification of Morbidity and Mortality After Thoracic Surgery. Ann Thorac Surg 2010;90:936-42. [Crossref] [PubMed]
- Kajiwara N, Kakihana M, Usuda J, et al. Extended indications for robotic surgery for posterior mediastinal tumors. Asian Cardiovasc Thorac Ann 2012;20:308-13. [Crossref] [PubMed]
- DeRose JJ Jr, Swistel DG, Safavi A, et al. Mediastinal mass evaluation using advanced robotic techniques. Ann Thorac Surg 2003;75:571-3. [Crossref] [PubMed]
- Bodner J, Wykypiel H, Greiner A, et al. Early experience with robot-assisted surgery for mediastinal masses. Ann Thorac Surg 2004;78:259-65. [Crossref] [PubMed]
- Savitt MA, Gao G, Furnary AP, et al. Application of robotic-assisted techniques to the surgical evaluation and treatment of the anterior mediastinum. Ann Thorac Surg 2005;79:450-5. [Crossref] [PubMed]
- Rueckert J, Swierzy M, Badakhshi H, et al. Robotic-assisted thymectomy: surgical procedure and results. Thorac Cardiovasc Surg 2015;63:194-200. [Crossref] [PubMed]