Pulmonary coin lesion mimicking lung cancer reveals an unexpected finding: Dirofilaria immitis
Introduction
A solitary pulmonary coin lesion can be attributed to a great variety of diseases. One of the extremely rare etiologies of the solitary pulmonary coin lesion is the human pulmonary dirofilariasis (HPD). HPD is attributed to an infection of the parasite Dirofilaria (D.) and especially to the type Dirofilaria immitis (DI). D. is a nematode that is also known as dog heartworm since it parasites in dog’s heart. Through mosquito vectors this parasite is transferred to humans. D. normally does not survive in the subcutaneous tissue. However, occasionally it is able to enter the blood circulation and from there the right heart ventricle. There it develops into a sexually immature worm. When its death occurs, it is washed into the pulmonary arteries and embolised a peripheral artery (1,2). This embolised distal pulmonary artery results in a pulmonary nodule around which granulation tissue with a central necrosis evolves (2). We present the case of a 62-year-old patient that presented in our clinic with a pulmonary nodule at the right lower lobe with suspicion of malignancy. However, the video-assisted thoracic surgical (VATS) wedge resection revealed this unexpected finding.
Case presentation
Medical history
A 62-year-old female was referred to our hospital after a routine check-up due to abnormal findings in chest X-ray. The patient had no significant past medical history other than a trigeminal neuralgia.
Preoperative assessment
The clinical examination revealed no abnormalities. Complete blood count showed white blood cell 4.97×103/µL, hemoglobin concentration 13.2 g/dL and platelet count 276×103/µL. Serum sodium, potassium, creatinine, calcium und liver function test were all within normal limits. Spirometry was normal with FEV1 2.42 L (102% of predicted), VC 3.00 L (102% of predicted). The blood gas analysis revealed no abnormality with PaCO2: 35 mmHg, PaO2: 74 mmHg and pH: 7.39.
The chest CT showed a 1.54 cm × 1.46 cm sized pulmonary nodule at the apical segment of the right lower lobe with well-defined nodule margins (Figure 1). On positron emission tomography-computed tomography (PET/CT) the pulmonary nodule showed no fludeoxyglucose (FDG) uptake. For further characterization a VATS wedge resection of the nodule with intraoperative frozen section was done.
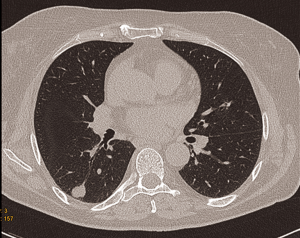
Procedure
The preoperative bronchoscopy showed no pathological findings. The nodule was removed completely with video-assisted thoracoscopic wedge resection. The inspection of the pleural cavity showed no inflammatory adhesions. The intraoperative frozen section showed no malignancy, but an old tissue necrosis. A chest drain was inserted before closing the chest. The tracheal extubation of the patient was performed with no difficulty. The patient was transferred postoperatively at the clinic’s intermediate care unit. The chest drain tube was removed on the first postoperative day. The further postoperative course was unproblematic. The patient was discharged on the third postoperative day. The further follow-up was uneventful. The histopathologic examination showed a 1.5 cm × 1.2 cm × 1.2 cm complete resected encapsuled nodule with a granulomatous inflammatory peripheral wall with a necrotic center (Figure 2). Isolated eosinophil cells were seen in the periphery of the nodule. The nodule was surrounded by normal lung parenchyma. The visceral pleura was intact. Thread worm parts were detected in a small artery in the nodule’s necrotic area. Based on its morphological features the worm was identified as DI. After these findings, no further medical treatment was suggested.
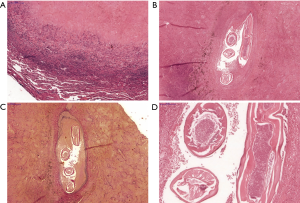
Discussion
The HPD is an extremely rare medical entity. To our knowledge less than 400 HPD cases have been described in the current literature (1-4). Except from the DI, there are also other types of D., for example, the D. repens that can cause subcutaneous and ocular lesion. Dirofilariasis of other organs has also been reported. Cardiovascular dirofilariasis, visceral dirofilariasis, in organs such as liver, uterus or dirofilariasis of the abdominal cavity have been observed. Generally, the disease can be distinguished in pulmonary D. and extra-pulmonary (3).
Regarding the geographic range of the disease cases of HPD have been reported in the west coast of the United States of America, the Gulf of Mexico, Brazil and Japan (1-3). However, as far as the USA is concerned it is believed that this clinical entity exists also in the other states of the country (1). Concerning Europe, HPD is very rare although the prevalence of cardiopulmonary dirofilariasis in dogs is higher in southern European countries than in northern ones. However, it seems that this has changed and the prevalence of this animal disease has increased in central and northern European countries in the last ten years and possibly that will also affect the human population (5). Predisposing or risk factors for this clinical entity are not clear and well defined. The ownership of a dog appears to be no serious factor. The number of the dog population in the area, the prevalence of the infection in this population, the number of infected mosquitos and the exposure of the human population to them can possibly contribute in the spread of the disease in a specific geographic area (2). It is also believed that the risk of HPD spreading is higher in periods of natural disasters (6).
The HPD is diagnosed usually in middle aged groups (between forty and sixty). A possible explanation could be that young people undergo imaging examination less frequently as older ones (1,2). The clinical symptomatology of this clinical entity is not specific. The majority of patients, as in our case, are asymptomatic and the only clinical presentation of HPD is a subpleural pulmonary coin lesion. However, in some cases at the time of diagnosis dyspnea, chest pain, hemoptysis and cold sweats have been described (1-3). This symptomatology is possibly attributed to the worm’s death in the human heart, its passage in the pulmonary circulation and infraction of the distal pulmonary artery (1).
For the diagnosis of the HPD exist no specific radiological signs and there are no specific blood tests that can lead direct to the disease’s diagnosis. In HPD eosinophilia is rare as it appears only in 17% of patients (7). In addition, serological tests for DI antigens are non-specific and have a low sensitivity as there is a cross reactivity with other parasites (1). As in our case, in the majority of patients a pulmonary lesion is randomly diagnosed by a radiological control (1-3). Usually in the chest X ray a subpleural pulmonary nodule sized 1–3 cm with well-defined margins is diagnosed. The lesions are usually detected at the right lower lobe (1,8). However, the pulmonary nodules can be multiple or bilateral (3). A chest CT proves the well-defined pulmonary nodule in the majority of cases (1,2). Other possible radiological findings in the CT could be pulmonary infiltrates, pleural thickening and pleural effusion (2). These HPD non-specific radiological imaging signs can cause significant differential diagnostic concerns as HPD nodules can be mimicking primary lung cancers or metastatic lung tumors leading to unnecessary diagnostic interventions (1,2). This clinical suspicion for a possible malignancy can be naturally higher in patents who are smokers or have symptoms similar to lung cancer. For example, Rodrigues-Silva et al reported that 70% of his patients with HPD were heavy smokers (2). Although, in an asymptomatic patient with a pulmonary nodule smaller than 3 cm HPD could be included in the differential diagnosis (1). Because of the many causes of a pulmonary nodule such as for example carcinoma, tuberculosis, fungal infections and hamartomas further radiological examinations is required (1,2). By suspicion of a malignancy in our patient a PET/CT was performed. However, this examination showed no uptake of 18-fluorodeoxyglucose (18-FDG). Stone et al. and Kang et al. reported each of one case of HPD that both showed a mild uptake of 18-FDG (4,8). Other examination, such as angiography of the right side of the heart and pulmonary artery, transthoracic needle aspiration, examination of bronchial washings and biopsy or sputum cytology do not assist the diagnosis of the disease (1). However, a case of HPD was diagnosed through CT-guided percutaneous needle lung biopsy, while another one was diagnosed with fine needle aspiration (1,9).
The definite diagnosis of the HPD is made through the wedge resection of the nodule (1,3,7,8). Macroscopically, the resected histological specimen consists of a spherical centrally necrotic nodule 1–3 cm in diameter that is surrounded by a granulomatous border and normal lung parenchyma. The nodule’s central necrotic area that is attributed to the DI embolism of the peripheral pulmonary artery (1,3). By these findings it is suggested that the histological specimen should be examined also with DNA PCR and ELISA in order to avoid the non-performance diagnosing DI (10).
The surgical wedge resection of the nodule is regarded as diagnostic and additionally curative (1,3,4,10). No serious complications have been reported by the lung operations for HPD except a case of reoperation because of postoperative bleeding associated with dense pleural adhesions. Regarding the treatment of the HPD it is believed that because the human body is a dead-end host for the DI and as there is no microfilaremia, no further medical cure is necessary. It is also believed that if the HPD could be diagnosed without an operation the surgical intervention would be unnecessary (2). A VATS is performed in the majority of cases, as in our one, in the context of the differential diagnosis of a lung cancer (1,2). Patients that were heavy smokers in the past and were unknown DI hosts can be falsely candidates for a more extensive operation (2). After the VATS resection and diagnosis of HPD no other therapeutic treatment is needed (1,2,10). However, other approaches in the diagnosis and treatment of this extremely rare clinical entity have been described. Magono et al. have reported a case of a patient that was treated without an operation and the diagnosis of HPD was reached through CT-guided percutaneous needle lung biopsy and immunological tests (9). However, Jelinek et al. have proposed an addition al diethylcarbamazine over a period of 4 weeks after the HPD surgical resection (11).
In conclusion, we believe that the HPD, as any other solitary peripheral pulmonary nodule of unknown etiology can easily and safely be diagnosed and treated by a VATS wedge resection.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Asimacopoulos PJ, Katras A, Christie B. Pulmonary dirofilariasis. The largest single-hospital experience. Chest 1992;102:851-5. [Crossref] [PubMed]
- Milanez de Campos JR, Barbas CS, Filomeno LT, et al. Human pulmonary dirofilariasis: analysis of 24 cases from São Paulo, Brazil. Chest 1997;112:729-33. [Crossref] [PubMed]
- Akao N. Human dirofilariasis in Japan. Trop Med Health 2011;39:65-71. [PubMed]
- Kang HJ, Park YS, Lee CH, et al. A case of human pulmonary dirofilariasis in a 48-year-old Korean man. Korean J Parasitol 2013;51:569-72. [Crossref] [PubMed]
- Morchón R, Carretón E, González-Miguel J, et al. Heartworm Disease (Dirofilaria immitis) and Their Vectors in Europe - New Distribution Trends. Front Physiol 2012;3:196. [Crossref] [PubMed]
- Simón F, Siles-Lucas M, Morchón R, et al. Human and animal dirofilariasis: the emergence of a zoonotic mosaic. Clin Microbiol Rev 2012;25:507-44. [Crossref] [PubMed]
- Ro JY, Tsakalakis PJ, White VA, et al. Pulmonary dirofilariasis: the great imitator of primary or metastatic lung tumor. A clinicopathologic analysis of seven cases and a review of the literature. Hum Pathol 1989;20:69-76. [Crossref] [PubMed]
- Stone M, Dalal I, Stone C, et al. 18-FDG Uptake in Pulmonary Dirofilariasis. J Radiol Case Rep 2015;9:28-33. [Crossref] [PubMed]
- Magono N, Yosimatu H, Suzuki Y, et al. A case of pulmonary dirofilariasis diagnosed by biopsy, immunological tests and the clinical course without operation. Nihon Kokyuki Gakkai Zasshi 2009;47:467-70. [PubMed]
- Biswas A, Reilly P, Perez A 4th, et al. Human pulmonary dirofilariasis presenting as a solitary pulmonary nodule: A case report and a brief review of literature. Respir Med Case Rep 2013;10:40-2. [Crossref] [PubMed]
- Jelinek T, Schulte-Hillen J, Löscher T. Human dirofilariasis. Int J Dermatol 1996;35:872-5. [Crossref] [PubMed]


