Impact of major bleeding on the risk of acute kidney injury in patients undergoing off-pump coronary artery bypass grafting
Introduction
Approximately 12% of acute coronary syndrome (ACS) patients require coronary artery bypass graft (CABG) during the index hospitalization (1,2). However, major bleeding is a common complication after CABG which impairs the clinical outcomes (3,4). Some studies demonstrated that patients with perioperative bleeding were susceptible to major adverse events including death, stroke and acute kidney injury (AKI) after CABG (5-7). AKI after CABG, in particular, was proposed to increase the short- and long-term risk of adverse outcomes such as myocardial infarction and death (8,9).
In recent years, the technique of off-pump CABG (OPCAB) has emerged to decrease perioperative complications such as bleeding by avoiding the use of cardiopulmonary bypass and the cross-clamping of the aorta (10). OPCAB has constituted about 15–20% of CABG in Europe and USA. However, it accounts for 60–100% of CABG surgery in Asian countries (11,12). The relationship between perioperative bleeding of OPCAB and AKI still remains unknown. Therefore, the purpose of this study is to evaluate the impact of perioperative bleeding on the risk of postoperative AKI in patient undergoing OPCAB.
Methods
Study patients and data collection
The present study is a retrospective, single-center, cohort analysis enrolling ACS patients undergoing OPCAB from February 2008 to December 2014 in university teaching hospital (Beijing Anzhen hospital). Among all ACS patients who underwent OPCAB, only those with sufficient records to specify bleeding events were eligible to be included. Patients were excluded for the following reasons: any contraindication to OPCAB, preoperative exposure to warfarin, preoperative severe renal insufficient and other open-chest surgeries including valvular repair or replacement. Informed consent was obtained from each patient on the day of admission. The ethical review and informed consent of this study were approved by institutional ethics committee of Beijing Anzhen Hospital, Capital Medical University (No. 2018011X).
To ensure the accuracy of data collection, the data was entered by two trained medical staffs and the important data information was monitored and revised by a third medical staff. Complete pre-, intra-, and postoperative data were available for all patients from an electronic database containing the baseline and operative data and immediate postoperative adverse events. Data on baseline characteristics, perioperative adverse events, perioperative hemoglobin (Hb) and creatinine levels, postoperative drainage output in 24 hours, pre- and postoperative CK-MB levels, and the amount of transfused blood product were collected retrospectively.
Definitions and study endpoints
Patients were divided into two groups according to the presence or absence of major bleeding. In the present study, we identified patients with major bleeding using the universal definition of perioperative bleeding (UDPB) criteria. Major bleeding was diagnosed in the presence of the UDPB class 3 and 4 defined as: (I) sternal closure delayed; (II) postoperative chest tube blood loss within 12 hours ≥1001 mL; (III) requiring transfusion of ≥5 U packed red cells (PRBCs) or fresh frozen plasma; (IV) surgical reexploration (4). Moreover, we calculated major bleeding rates using another two definitions including the Platelet Inhibition and Patient Outcomes (PLATO) and the Bleeding Academic Research Consortium (BARC) type 4 (13,14).
The primary endpoint was the occurrence of acute kidney injury (AKI). AKI is defined as an acute postoperative renal insufficiency resulting in one or more of the following in the first 48 hours after surgery as proposed by Acute Kidney Injury Network (AKIN) as stage 1 of acute kidney injury: (I) an increase of serum creatinine level of ≥0.3 mg/dL (≥26.4 µmol/L); (II) at least a 50% greater increase in creatinine above baseline preoperative level (1.5-fold from baseline); (III) a reduction in urine output (documented oliguria of less than 0.5 mL/kg per hour for more than six hours) (15). The secondary endpoints were mortality during hospitalization and postoperative MI (16).
Intra- and post-operative blood product transfusion rates and amounts were recorded for principal blood product types, including PRBC, platelets and fresh frozen plasma (FFP). In general, red blood cells (RBCs) were given intraoperatively to maintain a Hb concentration of more than 7 g/dL or a haematocrit (Hct) level of more than 20% or given postoperatively when Hb level was less than 8 g/dL. The need for additional blood product transfusion such as FFP an platelets (PLTs) remained at the discretion of the individual surgeon, anaesthesiologist or intensivist on a patient-by-patient basis.
Statistical analysis
Statistical analysis was performed using the SPSS version 24.0 statistical software (IBM Corporation, Armonk, New York, USA). Baseline characteristics were compared between the patients with major bleeding and without major bleeding. Continuous variables were expressed as mean value ± standard deviation and compared by the Student t-test if normally distributed and otherwise as median (minimum, maximum) and compared by the Wilcoxon rank sum test. Categorical variables are expressed as percentages and were compared by the χ2 statistic or continuity-correction χ2 when cell counts were <5 or Fisher’s exact test when cell counts were <1.
We used multivariable logistic regression analysis to investigate the association between perioperative bleeding and postoperative AKI adjusting for potential confounding factors. Forward stepwise selection was used to identify significant confounding variables. Potential confounders which had been reported in previous studies as important determinants of perioperative outcomes would be offered to the logistic regression models including: age, gender, body mass index (BMI), diabetes, hypertension, prior myocardial infarction (MI), renal insufficiency, decreased left ventricular ejection fraction (LVEF), systolic blood pressure (SBP) values, preoperative Hct values and discontinuation of clopidogrel. Renal insufficiency for baseline characteristics was defined as preoperative creatinine clearance rate (Ccr) values < 60 mL/min. And closely associated factors (P<0.10) from the univariate analysis were also included in the multivariable logistic regression analysis (P<0.05 was retention criterion for each factor). Model fit analysis was evaluated using the Hosmer and Lemeshow goodness-of-fit statistic. Area below ROC curve was reported to have predictive power. Power of the association between risk factors and outcomes was expressed as odds ratio (OR).
All P values are 2-sided. Results were considered to be statistically significant at a P<0.05.
Results
Demographic and perioperative characteristics
Among a total of 4,030 ACS patients included in this study, 394 (9.8%) experienced the UCPB class 3–4 bleeding. In addition, PLATO major life-threatening bleeding was observed in 1,459 (36.2%) patients and BARC type 4 bleeding in 170 (4.2%) patients during perioperative period. AKI was found in 995 (24.7%) patients and 52 (1.29%) required renal replacement therapy (RRT). In the overall population, the mean age was 62.1 years and 76.2% were males. The mean baseline creatinine clearance was 88.78 mL/min. There were 120 (2.98%) deaths and 201 (4.99%) postoperative MIs during hospitalization. Moreover, there were 1,491 (37%) patients who received clopidogrel within 5 days before OPCAB surgery
Demographic and preoperative characteristics of subjects and perioperative outcomes are presented in Table 1. The patients were divided into two groups according to the presence or absence of major bleeding. A higher proportion of patients with major bleeding had lower BMI (52.54% vs. 43.23%, P<0.001) and with clopidogrel exposure within 5 days prior to surgery (54.57% vs. 35.09%, P<0.001) and were male gender (85.28% vs.75.30%, P<0.001) compared with patients without major bleeding. It is noteworthy that preoperative creatinine clearance was not statistically different between patients with and without bleeding. There was no major difference in age, LVEF, hypertension, diabetes, prior MI, preoperative haematocrit values, SBP, multivessel disease and number of distal anastomoses.
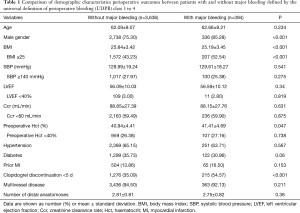
Full table
Effect of major bleeding on acute kidney injury
The primary endpoint occurred in 130 (32.99%) patients in major bleeding group and 865 (23.79%) in non-major bleeding group (P<0.001). Patients with major bleeding were at an higher risk of death (10.66% vs. 2.15%, P<0.001) and MI (7.87% vs. 4.65%, P=0.005) during hospitalization (Table 2).

Full table
Univariable and multivariable analysis were then performed to find possible predictive factors for occurrence of AKI using logistic regression. Table 3 outlines part of the results of univariable analysis. The unadjusted relative risk for AKI increased by 58% in patient with major bleeding (OR =1.58, 95% CI: 1.26 to 1.97, P<0.001) when compared with the patients without bleeding. And patient with advanced age, hypertension, diabetes, prior MI, lower Ccr, increased SBP or decreased haematocrit values were at an increased risk of AKI. Comparison of perioperative characteristic and incidence of major bleeding using different definitions between patients with and without AKI is displayed in Table 4. Rates and amount of postoperative PRBCs and FFP transfusion were significantly higher in patients with AKI. Among decrease in hemoglobin, transfusion and chest tube output which compose the definition of major bleeding, transfusion was identified as the most significant risk factor for AKI according to univariable analysis (OR =2.13 95% CI: 1.42 to 3.19, P<0.001). Furthermore, rates of major bleeding defined by all three definitions were significantly increased in patients with postoperative AKI.
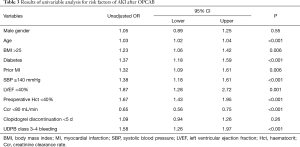
Full table

Full table
In multivariable logistic regression analysis, variables independently associated with AKI after OPCAB are shown in Table 5. Logistic regression model confirmed that AKI was strongly associated with perioperative major bleeding with an odds ratio of 1.67 (95% CI: 1.32 to 2.10, P<0.001). The AUC (area under the curve) of this regression model was 0.76 (95% CI: 0.83–0.78) showing that the UDPB classification had predictive value of AKI. Advanced age, higher BMI, diabetes, prior MI, increased SBP, impaired LVEF, decreased Ccr baseline and decreased haematocrit were independent risk factors of postoperative AKI. When decrease in hemoglobin, transfusion and chest tube output were included in the same regression model to replace major bleeding individually, transfusion was most strongly associated with AKI after surgery (OR =2.08, 95% CI: 1.38–3.16, P=0.010) (Table 6).

Full table

Full table
Discussion
This present study would suggest that perioperative major bleeding is associated with a significant higher risk of AKI after OPCAB. Blood and blood products transfusion significantly correlated with AKI after OPCAB. Thus, preventing excessive bleeding and minimizing transfusion during perioperative period can reduce the risk of AKI and improve clinical outcomes of patients undergoing OPCAB.
Perioperative severe bleeding and the related need of transfusion are known to increase the risk of adverse outcomes (17). Our results are in conformity with some previous published studies reporting on the association between an increased risk of adverse clinical outcomes and periprocedural bleeding and transfusion (18). The rate of perioperative major bleeding in patients undergoing CABG varied between different studies due to different definitions of bleedings. Kinnunen et al. observed that the relation between adverse outcomes including acute kidney injury and bleeding also existed in low-risk patients with EuroSCORE II of less than 2% (19). Another prospective study of Kinnunen et al. and a retrospective study of Paone et al. described that even minor bleeding and subsequent blood transfusion (1–2 units) was also associated with an increased risk of AKI as well as other adverse outcomes (5,20). Conversely, analysis from ACUITY trial by Stone et al. found that RBC transfusion of ≥4 U after CABG was strongly associated with subsequent mortality but transfusion of 1 to 3 U of RBCs may not carry the same negative prognostic implications (7). The results from a prospective study conducted by Biancari et al. provided evidence on the impact of bleeding and transfusion on the development of neurological impairment after CABG and reduction of blood loss might decrease the risk of severe complications after surgery. However, few studies have examined the relationship between periprocedural bleeding or transfusion and AKI incidence in patients who has undergone OPCAB which is more common in Asians and might bring some early benefit by avoiding the conventional on-pump technique. Furthermore, the bleeding definitions that were used varied across previous studies. Our retrospective study, by contrast, enrolled patients who underwent OPCAB and suggested that perioperative bleeding defined by the UDPB class 3 to 4 increases the risk of postoperative AKI significantly.
The association between blood transfusion and AKI may be due to either a direct transfusion-related kidney injury, or an indirect effect in which the transfusion is acting as a surrogate marker for hypotension or decreased oxygen delivery (21). The mechanisms of AKI after transfusion have not been clearly defined, but there were several potential pathophysiological causes (22). The impairment of tissue oxygen delivery and predisposition to inflammatory response and oxidative stress promoted by transfusion might play an important role in organ injury (23). Red blood corpuscles undergo irreversible structural and functional changes during storage under hypothermic conditions, then predispose to tissue hypoxia and cause toxic injury (22).
Perioperative bleeding is common among patients undergoing cardiac surgery. However, the definitions of major bleeding and the stratifications of its severity are variable across different studies. Dyke et al. proposed the universal definition of perioperative bleeding (UDPB) as a classification for the severity of perioperative bleeding in patients undergoing adult cardiac surgery (4). The rate of major bleeding in the study of Dyke et al. was 9.6% and was similar with the results of our study in which the rate of major bleeding was 9.8%. Kinnunen et al. validated the UDPB classification in 2,764 patients undergoing CABG and their analysis showed that the UDPB could effectively stratified the severity of bleeding (24). Therefore, the UDPB classification can be used as a promising research tool for appropriate stratification of the perioperative risk of bleeding and provide prognostic information. Even though we identified patients with major bleeding using the UDPB criteria in consideration of the surgical setting of our study, the PLATO major bleeding which was used in most nonsurgical clinical trials (25) and the BARC type 4 bleeding of which the incidence rate was very low (4.2%) were also more common in patient with AKI and can be used as predictors of AKI. In our study, part of patients underwent surgery with clopidogrel exposure <5 days prior to surgery, which might be the reason why the major bleeding rate was as high as reported in on-pump CABG cases.
Our present study has some important clinical implications. Firstly, reducing blood loss and minimizing transfusion can improve clinical outcomes of patients undergoing OPCAB by potentially decreasing the risk of postoperative AKI. AKI is a common and serious complication of cardiac surgery (26). The incidences of AKI between 7% and 40% have been reported depending on the definition used (27-30). The rate of postoperative AKI defined by AKIN among OPCAB patients in a previous study of Lamy et al. (28%) was in accordance with our study (24.7%) (10). In the previous literature, AKI after cardiac surgery was reported to be associated with a short- and long-term increased risk of poor prognosis. Ryden et al. demonstrated that AKI after CABG resulted in an increased long-term risk of MI and dearth and this was true even for small increases in Ccr after surgery (9). A study from Sweden including 24,018 patients identified AKI to be associated with increased long-term risk of heart failure after CABG indicating that patients with AKI after CABG should be followed closely to detect early changes in cardiac function (31). Another large nationwide study in Sweden by Holzmann et al. found that AKI was associated with a higher long-term risk of stroke in patients younger than 65 years (32). Bahar et al. explored the risk factors and prognosis of acute renal failure following open heart surgery. The results supported that bleeding in the early postoperative period may precipitate renal failure by prerenal mechanisms and acute renal failure after cardiac surgery often resulted in high morbidity and mortality (8). In consideration of that AKI after cardiac surgery increases the risk of adverse outcomes and major bleeding is strongly associated with higher incidence of AKI after OPCAB, prevention of perioperative excessive bleeding may improve the outcome of OPCAB. Similarly, liberal use of blood transfusion may expose patients to risk of AKI therefore minimizing perioperative transfusion may also bring better outcomes of OPCAB. Secondly, several previous trails suggested that the use of off-pump technique in coronary artery bypass surgery can reduce incidence of AKI compared with conventional on-pump surgery (33). Furthermore, the results of this study showed that elderly, higher BMI, hypertension, diabetes, prior MI and decreased Hct were independent risk factors for AKI after surgery. Accordingly, patients with above risk factors tend to be at a higher risk of adverse outcomes and are recommended to choose OPCAB.
Limitation
Several limitations of this study should be acknowledged. First, the indications for transfusion after OPCAB were not prespecified and differences in transfusion threshold may exist between surgical teams. Second, our study may have potential bias due to the absence of data regarding the influence of possible confounding variables that affect kidney function intra- and post-operative hemodynamics, the priming volume, vasopressor deses, perioperative fluid balance or differences in the dose of colloids, thus we could not confirm if hypotension was responsible for the development of AKI. Third, part of patients who received one or two units transfusion also suffered from severe perioperative bleeding, therefore we could not distinguish the effect of RBC transfusion or bleeding-related anemia on the clinical outcomes.
Conclusions
In conclusion, the present study demonstrated a strong independent relationship between perioperative bleeding and AKI after OPCAB. These data suggest that transfusion has the most significant implications causing an increased risk of AKI after surgery. Our study indicates that minimizing bleeding and blood transfusion to avoid AKI after OPCAB may improve the outcomes after surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Informed consent was obtained from each patient on the day of admission. The ethical review and informed consent of this study were approved by institutional ethics committee of Beijing Anzhen Hospital, Capital Medical University (No. 2018011X).
References
- Dyke CM, Bhatia D, Lorenz TJ, et al. Immediate coronary artery bypass surgery after platelet inhibition with eptifibatide: results from PURSUIT. Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrelin Therapy. Ann Thorac Surg 2000;70:866-71; discussion 871-2. [Crossref] [PubMed]
- Fox KA, Anderson FA Jr, Dabbous OH, et al. Intervention in acute coronary syndromes: do patients undergo intervention on the basis of their risk characteristics? The Global Registry of Acute Coronary Events (GRACE). Heart 2007;93:177-82. [Crossref] [PubMed]
- Hansson EC, Rexius H, Dellborg M, et al. Coronary artery bypass grafting-related bleeding complications in real-life acute coronary syndrome patients treated with clopidogrel or ticagrelor. Eur J Cardiothorac Surg 2014;46:699-705; discussion 705. [Crossref] [PubMed]
- Dyke C, Aronson S, Dietrich W, et al. Universal definition of perioperative bleeding in adult cardiac surgery. J Thorac Cardiovasc Surg 2014;147:1458-63.e1. [Crossref] [PubMed]
- Kinnunen EM, Zanobini M, Onorati F, et al. The impact of minor blood transfusion on the outcome after coronary artery bypass grafting. J Crit Care 2017;40:207-12. [Crossref] [PubMed]
- Biancari F, Tauriainen T, Perrotti A, et al. Bleeding, transfusion and the risk of stroke after coronary surgery: A prospective cohort study of 2357 patients. Int J Surg 2016;32:50-7. [Crossref] [PubMed]
- Stone GW, Clayton TC, Mehran R, et al. Impact of major bleeding and blood transfusions after cardiac surgery: analysis from the Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial. Am Heart J 2012;163:522-9. [Crossref] [PubMed]
- Bahar I, Akgul A, Ozatik MA, et al. Acute renal failure following open heart surgery: risk factors and prognosis. Perfusion 2005;20:317-22. [Crossref] [PubMed]
- Ryden L, Ahnve S, Bell M, et al. Acute kidney injury after coronary artery bypass grafting and long-term risk of myocardial infarction and death. Int J Cardiol 2014;172:190-5. [Crossref] [PubMed]
- Lamy A, Devereaux PJ, Prabhakaran D, et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med 2012;366:1489-97. [Crossref] [PubMed]
- Taggart DP, Altman DG. Off-pump vs. on-pump CABG: are we any closer to a resolution? Eur Heart J 2012;33:1181-3. [Crossref] [PubMed]
- Yaku H, Doi K, Okawa K. Off-pump coronary artery bypass grafting revisited: experience and evidence from Japan. Ann Thorac Cardiovasc Surg 2013;19:83-94. [Crossref] [PubMed]
- Brascia D, Reichart D, Onorati F, et al. Validation of Bleeding Classifications in Coronary Artery Bypass Grafting. Am J Cardiol 2017;119:727-33. [Crossref] [PubMed]
- Vranckx P, White HD, Huang Z, et al. Validation of BARC Bleeding Criteria in Patients With Acute Coronary Syndromes: The TRACER Trial. J Am Coll Cardiol 2016;67:2135-44. [Crossref] [PubMed]
- Pereira M, Rodrigues N, Godinho I, et al. Acute kidney injury in patients with severe sepsis or septic shock: a comparison between the 'Risk, Injury, Failure, Loss of kidney function, End-stage kidney disease' (RIFLE), Acute Kidney Injury Network (AKIN) and Kidney Disease: Improving Global Outcomes (KDIGO) classifications. Clin Kidney J 2017;10:332-40. [PubMed]
- Ebrahimi R, Dyke C, Mehran R, et al. Outcomes following pre-operative clopidogrel administration in patients with acute coronary syndromes undergoing coronary artery bypass surgery: the ACUITY (Acute Catheterization and Urgent Intervention Triage strategY) trial. J Am Coll Cardiol 2009;53:1965-72. [Crossref] [PubMed]
- Loor G, Rajeswaran J, Li L, et al. The least of 3 evils: exposure to red blood cell transfusion, anemia, or both? J Thorac Cardiovasc Surg 2013;146:1480-7.e6. [Crossref] [PubMed]
- Murphy GJ, Reeves BC, Rogers CA, et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation 2007;116:2544-52. [Crossref] [PubMed]
- Kinnunen EM, De Feo M, Reichart D, et al. Incidence and prognostic impact of bleeding and transfusion after coronary surgery in low-risk patients. Transfusion 2017;57:178-86. [Crossref] [PubMed]
- Paone G, Likosky DS, Brewer R, et al. Transfusion of 1 and 2 units of red blood cells is associated with increased morbidity and mortality. Ann Thorac Surg 2014;97:87-93; discussion 93-4. [Crossref] [PubMed]
- Khan UA, Coca SG, Hong K, et al. Blood transfusions are associated with urinary biomarkers of kidney injury in cardiac surgery. J Thorac Cardiovasc Surg 2014;148:726-32. [Crossref] [PubMed]
- Karrowni W, Vora AN, Dai D, et al. Blood transfusion and the risk of acute kidney injury among patients with acute coronary syndrome undergoing percutaneous coronary intervention. Circ Cardiovasc Interv 2016.9. [PubMed]
- Karkouti K. Transfusion and risk of acute kidney injury in cardiac surgery. Br J Anaesth 2012;109 Suppl 1:i29-38. [Crossref] [PubMed]
- Kinnunen EM, Juvonen T, Airaksinen KE, et al. Clinical significance and determinants of the universal definition of perioperative bleeding classification in patients undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg 2014;148:1640-6.e2. [Crossref] [PubMed]
- Becker RC, Bassand JP, Budaj A, et al. Bleeding complications with the P2Y12 receptor antagonists clopidogrel and ticagrelor in the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J 2011;32:2933-44. [Crossref] [PubMed]
- Dardashti A, Ederoth P, Algotsson L, et al. Incidence, dynamics, and prognostic value of acute kidney injury for death after cardiac surgery. J Thorac Cardiovasc Surg 2014;147:800-7. [Crossref] [PubMed]
- Mehta RH, Honeycutt E, Patel UD, et al. Impact of recovery of renal function on long-term mortality after coronary artery bypass grafting. Am J Cardiol 2010;106:1728-34. [Crossref] [PubMed]
- Brown JR, Kramer RS, Coca SG, et al. Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann Thorac Surg 2010;90:1142-8. [Crossref] [PubMed]
- Khilji SA, Khan AH. Acute renal failure after cardiopulmonary bypass surgery. J Ayub Med Coll Abbottabad 2004;16:25-8. [PubMed]
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006;1:19-32. [Crossref] [PubMed]
- Olsson D, Sartipy U, Braunschweig F, et al. Acute kidney injury following coronary artery bypass surgery and long-term risk of heart failure. Circ Heart Fail 2013;6:83-90. [Crossref] [PubMed]
- Holzmann MJ, Ahlback E, Jeppsson A, et al. Renal dysfunction and long-term risk of ischemic and hemorrhagic stroke following coronary artery bypass grafting. Int J Cardiol 2013;168:1137-42. [Crossref] [PubMed]
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009;119:495-502. [Crossref] [PubMed]


