A case report on parainfluenza virus type 4a infection in a 1-year-old boy with biphasic fever
Introduction
Pediatricians sometimes encounter cases that showed a symptom of fever with two distinct peaks (biphasic fever) in the clinical course of patients with influenza (1-3). However, it is not well understood how an infection with influenza virus (IFV) alone causes it. Occurrence of such a fever pattern in infection with PIVs 1, 2, 3 and respiratory syncytial virus (RSV) was recorded in the 1980s on the hospitalized pediatric patients (4) but, thereafter, has not been well-studied. In outpatient clinics, it is important to determine further drug prescription, whether the biphasic fever occurs by pure viral infection without involvement of the effect of medication or by other pathogens. We wanted to clarify whether any respiratory virus other than the one that caused the first fever peak are involved in the second peak or not, but we do not have a general conclusion for now. We had been conducting virological investigations since 2012 on pediatric patients who had influenza-like illness and visited clinics. We present here a case of a boy who showed biphasic fever in the course of upper respiratory disease with long-lasting cough. The possibility of single infection with parainfluenza virus type 4a (PIV4a) was strongly suggested with virological evidence through viral isolation and PCR analyses.
Case presentation
A 1-year-old boy experiencing fever of 38.2 °C, slight cough, and uneasiness visited his home doctor on January 26, 2016. The result of the rapid IFV-antigen test (Immuno Ace Flu®, Tauns, Izunokuni, Japan) performed on his nasal aspirate was negative. Neither anti-influenza drug nor antibiotics was prescribed, and careful follow-up was advised. His body temperature returned to normal at 36.5 °C on the next day, but the fever rose again on day 3, and the temperature peaked at 39.6 °C on day 4.
During the recurrent fever, his nasal specimen was collected, and the specimen was tested for rapid IFV-antigen. However, the result was negative again. The fever started to decline after the peak, and one and a half days after the onset of the recurred fever, his body temperature became close to normal at 37 °C.
Aside from having fever, the patient had persistent cough from days 1 to 9, which started with the fever and gradually augmented. The cough continued even during the intermittent phase of the fever between days 2 and 3, and until 3 days after resolution of the fever and gradually disappeared (Figure 1).
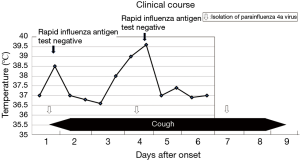
Microbiological analyses
The nasal aspirate specimens obtained on days 1, 4, and 7 were inoculated into six kinds of cells (HFL-III, HEp-2, Vero, MDCK, LLC-MK2, and MNT-1). These cell culture systems are optimized for isolation of various kinds of viruses, including IFV types A, B, C, PIV types 1, 2, 3, 4, RSV, human metapneumovirus, mumps virus, enteroviruses, rhinovirus, adenovirus, and herpes virus (5). Cell fusion appeared in LLC-MK2 cells inoculated with all the specimens obtained at days 1, 4, 7 under microscopic investigation on days 7, 2, and 5 after inoculation, respectively. Occurrence of the multinuclear giant cell formation by cell fusion is the cytopathic effect (CPE) characteristic for PIV4 multiplication (6-8). Final identification of PIV4a isolation was confirmed by the detection of the viral NP gene using conventional RT-PCR (6) from the culture medium of the LLC-MK2 cells that showed CPE. Meanwhile, none of the other viruses except for PIV4 were isolated in the cell systems. In addition, none of major respiratory viruses including RSV, PIV1-3, hMPV, IFV-A, B, AdV, RV and bocavirus was detected in the RNA extracted from the clinical specimens—using real-time PCR system of Cycler PCR® respiratory viruses detection kit Ver.3 (Takara Bio., Kusatsu, Japan) (9). Involvement of coronaviruses was excluded also by another real-time PCR system that was described previously (10,11). Those systems could detect the above-mentioned viruses at the detection limit of 10 gene copies/µL of clinical specimen. Therefore, PIV4a was strongly suggested as the causative agent of the patient’s illness.
Discussion
A case of infection caused by PIV4a that showed two distinct peaks in fever was described in this report. A possibility of bacterial involvement in the fever could be excluded, judging from the clinical course that the fever declined rapidly after its second peak without antibiotic treatment. The infection was determined by virus isolation and involvement of other major respiratory viruses with PIV4 in the illness (12) was almost excluded by the negative results in isolation in our virus isolation system and in viral gene-detection with multiplex real-time PCR, though the exclusion was not definitely conclusive since there were limitations in their competency in viral detection. Many reports of PIV4 infections have been reported so far from an epidemiological or clinical view point. They enumerated the symptoms of the infection including the frequency and/or duration of the fever, but they did not provide the information on the pattern of the fever nor the duration of the cough (8,12). Thus, this case report is significant as it describes details of a PIV4 infection.
Epidemiological and clinical studies on PIV4 infection have been mainly based on the detection of the viral gene in the clinical specimen using PCR (12-15), with some exceptions (8,16-18). We were able to isolate PIV4a from the specimens obtained on days 1, 4, and 7 that caused CPE in the LLC-MK2 cell on the 7th, 2nd, and 5th day after inoculation, respectively. If the duration from the inoculation to appearance of CPE inversely correlate to the load of active virus in the inoculated specimen as is frequently experienced in viral infection experiments, the duration difference might mean that active viral load in the nasal cavity was the highest on day 4 among those collected on three separate times. Those results were supported by quantitative analyses on the amounts of the virus in the specimens using a real-time PCR system (11), as well (data not shown). The isolation on day 7 would be consistent with the finding that the virus shedding in patients with PIV infections other than type 4 tends to linger long (13) and continue until even after the disappearance of the symptoms. This kind of viral shedding pattern is different from that of IFV infections, in which the shedding of active virus peaks at the initial phase of the illness and rapidly decreases thereafter (19). Understanding those pattern differences might be important for infection control of each viral infection. Analyses with PCR may provide information on virus accumulation in the specimens both of active and inactive viruses, but that of the active virus was possible in our study because the virus isolation was successful.
The biphasic fever itself was not rarely observed among pediatric influenza cases. It was reported that the cases with biphasic fever accounted for approximately 7% of all pediatric influenza cases in Kitakyushu City in 1986, without anti-influenza drug treatment (20). However, there was also a report that the frequency of biphasic fever declined after introduction of the neuraminidase inhibitor drug (21). In any case, the possibility that the biphasic fever during the winter season is caused by an infection with IFV is relatively high. Therefore, it might be reasonable that physicians tend to empirically prescribe neuraminidase inhibitor drugs at the second peak of the biphasic fever even if the result of the rapid IFV-antigen test is negative when influenza is circumstantially suspected (22). Our case would call for a caution that influenza-like cases caused by a respiratory virus other than IFV can also cause biphasic fever.
Viral isolation or gene analysis is not always available in many medical institutions. It would be of great importance for clinical information on various kinds of infections to be collected and consolidated, and easy and accurate methodologies with reasonable costs to identify causative agents to be developed. Fundamentally, the mechanisms of the occurrence of biphasic fever have not been clarified in many respiratory viral diseases including influenza, and even its frequency among the cases has been unclear. Basic data on the biphasic fever should be accumulated.
Acknowledgements
The authors greatly appreciate Drs. Kenji Takasaki, Yuji Yamashita, and Takahito Yokoyama for their helpful discussion, and Dr. Isolde Dapat and Enago (www.enago.jp) for English language review by a native speaker. This work was financially supported by the Clinical Research Division of Sendai Medical Center and the Research Program on Promoting Development of Innovative Drugs against Emerging and Reemerging Infectious Diseases from Japan Agency for Medical Research and Development, AMED.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: A written informed consent from his parent and an approval of the ethic committee of Sendai Medical Center were obtained for the presentation.
References
- Adams JM, Thigpen MO, Rickerd ER. An epidemic of influenza A in infants and children: A clinical and laboratory investigation. JAMA 1944;125:437-77.
- Sakuma T. Infant influenza. Acta Paediatr Jpn 1997;39:669-75. [Crossref] [PubMed]
- Koseki N, Kaiho M, Kikuta H, et al. Comparison of the clinical effectiveness of zanamivir and laninamivir octanoate for children with influenza A(H3N2) and B in the 2011-2012 season. Influenza Other Respir Viruses 2014;8:151-8. [Crossref] [PubMed]
- Putto A, Ruuskanen O, Meurman O. Fever in respiratory virus infections. Am J Dis Child. 1986;140:1159-63. [PubMed]
- Numazaki Y, Oshima T, Ohmi A, et al. A microplate method for isolation of viruses from infants and children with acute respiratory infections. Microbiol Immunol 1987;31:1085-95. [Crossref] [PubMed]
- Abiko C, Mizuta K, Aoki Y, et al. An outbreak of parainfluenza virus type 4 infections among children with acute respiratory infections during the 2011-2012 winter season in Yamagata, Japan. Jpn J Infect Dis 2013;66:76-8. [Crossref] [PubMed]
- Sato K, Watanabe O, Ohmiya S, et al. Efficient isolation of human parainfluenza viruses 1 and 3 using MNT-1, a human malignant melanoma cell line system that exhibits apparent cytopathic effect. Microbiol Immunol 2016;60:801-5. [Crossref] [PubMed]
- Billaud G, Morfin F, Vabret A, et al. Human parainfluenza virus type 4 infections: a report of 20 cases from 1998 to 2002. J Clin Virol 2005;34:48-51. [Crossref] [PubMed]
- Hamano-Hasegawa K, Morozumi M, Nakayama E, et al. Comprehensive detection of causative pathogens using a real-time OCR to diagnose pediatric community-acquired pneumonia. J Infect Chemother 2008;14:424-32. [Crossref] [PubMed]
- Gaunt ER, Hardie A, Claas EC, et al. Epidemiology and clinical presentations of the four Coronaviruses 229 E, HKU 1, NL63, and OC43 detected over 3 years using a novel multiples real time PCR method. J Clin Microbiol 2010;48:2940-7. [Crossref] [PubMed]
- Nishimura H, Sato K, Kadji FM, et al. Case study-based time-course analysis of symptoms of respiratory syncytial virus infections followed by acute sinusitis in otherwise-healthy adults. J Thorac Dis 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Ren L, Gonzalez R, Xie Z, et al. Human parainfluenza virus type 4 infection in Chinese children with lower respiratory tract infections: a comparison study. J Clin Virol 2011;51:209-12. [Crossref] [PubMed]
- Frost HM, Robinson CC, Dominguez SR. Epidemiology and clinical presentation of parainfluenza type 4 in children: a 3-year comparative study to parainfluenza types 1-3. J Infect Dis 2014;209:695-702. [Crossref] [PubMed]
- Lau SK, Li KS, Chau KY, et al. Clinical and molecular epidemiology of human parainfluenza virus 4 infections in Hong Kong: subtype 4B as common as subtype 4A. J Clin Microbiol 2009;47:1549-52. [Crossref] [PubMed]
- Fathima S, Simmonds K, Invik J, et al. Use of laboratory and administrative data to understand the potential impact of human parainfluenza virus 4 on cases of bronchiolitis, croup, and pneumonia in Alberta, Canada. BMC Infect Dis 2016;16:402. [Crossref] [PubMed]
- Tyrrell DA, Bynoe ML. Studies on Parainfluenza type 2 and 4 viruses obtained from patients with common colds. BMJ 1969;1:471-4. [Crossref] [PubMed]
- Lau SK, To W, Tse PW. Human parainfluenza virus-4 outbreak and the role of diagnostic tests. J Clin Microbiol 2005;43:4515-21. [Crossref] [PubMed]
- Vachon ML, Dionne N, Leblanc E, et al. Human parainfluenza type 4 infections, Canada. Emerg Infect Dis 2006;12:1755-8. [Crossref] [PubMed]
- Suess T, Remschmidt C, Schink SB, et al. Comparison of shedding characteristics of seasonal influenza virus (sub) types and influenza A (H1N1) pdm09; Germany, 2007-2011. PloS One 2012;7. [Crossref] [PubMed]
- Sakuma T. Influenza infection in a pediatric clinic. J Kitakyushu City Medical Association. 1986;30-8. (in Japanese).
- Suzuki E, Ichihara K. The course of fever following influenza virus infection in children treated with oseltamivir. J Med Virol 2008;80:1065-71. [Crossref] [PubMed]
- Wang K, Shun-Shin M, Gill P, et al. A. Neuraminidase inhibitors for preventing and treating influenza in children. Cochrane Database Syst Rev 2012;18:1.


