Perioperative outcome after open and thoracoscopic segmentectomy for the treatment of malignant and benign pulmonary lesions: a propensity-matched analysis
Introduction
Anatomic lobectomy is considered the standard of care treatment for early stage lung cancer (1,2). Given similar rates of morbidity and survival, segmentectomy may be a less invasive alternative in tumors of less than two centimeters in size (3-6). Nonetheless, sublobar resection is considered inadequate in larger lesions (1,2,7). In compromised patients, not suitable for lobectomy, however, segmentectomy might offer the best chance of curative resection. Also, in the treatment of centrally located or multiple metastases, where wedge resection would not achieve a complete resection, segmentectomy can provide radicality and preserve functional lung tissue (8-10).
Compared with open surgery, video-assisted thoracoscopic surgery (VATS) for the treatment of lung cancer is associated with less perioperative morbidity, less pain and shorter hospitalization without compromising oncological outcomes (11-13). Regarding the application of VATS techniques on anatomic segmentectomy, recent studies showed fewer pulmonary complications, decreased hospital length of stay and similar long-term survival compared with open segmentectomy (14-17). The validity of these results, however, is limited by small numbers of patients, lack of matching or use of registry data. Furthermore, most studies focused solely on lung cancer patients and early-stage tumors.
The aim of this study was to compare the perioperative outcome of patients receiving anatomic segmentectomy either by open surgery or VATS. To assess the short-term morbidity of the procedure itself, lung cancer patients in all stages as well as patients with pulmonary metastases and benign lesions scheduled for segmental resection were enrolled in this study.
Methods
Patients
Between January 2009 and December 2015, data were prospectively collected of 7,037 consecutive patients that underwent thoracic surgery at the Hospital of the City of Cologne. Patients’ characteristics, operative procedures and postoperative outcome were assessed with a standardized data entry form. The data of all patients treated with anatomic segmentectomy for lung cancer, pulmonary metastases or benign pulmonary lesions were identified and extracted from the hospital’s database. A total of 445 patients were included and retrospectively analyzed. The study protocol was approved by the Institutional Review Board of the Hospital of the City of Cologne, Germany (No. 126/2016). Informed consent was obtained from all patients.
All patients underwent preoperative tumor staging using PET-CT and magnetic resonance imaging (MRI) of the brain. In case of suspected N2-N3 disease endobronchial ultrasound (EBUS) bronchoscopy or mediastinoscopy was performed. The collected variables were the following: host factors were age, gender, smoking history, forced expiratory volume in 1 second (FEV1) and the diffusing capacity of the lung for carbon monoxide (DLCO). Pathological parameters were histological subtype, tumor size, pathological stage (pTNM) and radicality of resection. Operative variables were surgical technique (open vs. VATS segmentectomy) location of segmentectomy and duration of the procedure. The indication for open versus VATS segmentectomy was discussed in the interdisciplinary conference of the thoracic surgeons in our institution. All operations were conducted from five consulting thoracic surgeons in our affiliation. Collected postoperative data were time of hospitalization, chest tube duration and postoperative morbidity and mortality.
Operative techniques
All operations were performed under general anesthesia with one-lung ventilation. Open segmentectomy was performed by muscle-sparing anterolateral thoracotomy. VATS segmentectomy was performed either by single-, two- or three-port technique with a four-centimeters anterior access incision. In case of a multi-port access one or two additional one-centimeter incisions were performed. Anatomic segmentectomy was performed by dissection and division of the segmental vein, artery and bronchus. The parenchyma was divided after insufflation of the lung and macroscopic assessment of the intersegmental borders using stapling devices, bipolar scissors or high energy devices. In case of the use of bipolar scissors or high energy devices, parenchyma was dissected between the neighboring segmental veins and closure was achieved by running suture adapting the visceral pleura, taking caution of not entangling the neighboring vessels.
Follow-up
Postoperative complications were defined as deviation from the normal postoperative course within 30 days after surgery. Severe complications recorded were respiratory failure, pneumonia, myocardial infarction and postoperative stroke. Further complications noted were atrial fibrillation, prolonged air leak (>7 days), pneumothorax, postoperative bleeding, wound infection, abdominal complications (postoperative ileus, gastrointestinal bleeding or acute mesenteric ischemia) and recurrent laryngeal nerve injury.
Statistical analysis
Statistical evaluation was conducted with SPSS (IBM Corp., Released 2012, IBM SPSS Statistics for Windows, Version 21.0. Armonk, USA). The χ2-test was used for comparison of categorical variables. Comparison of means was performed by the unpaired t-test or Mann-Whitney U test as appropriate. P values <0.05 were considered significant.
To control for confounding influences a propensity score analysis using the SPSS custom dialog was conducted. In a first step the propensity score, i.e., the probability to be treated with VATS or open surgery, was estimated using logistic regression. The variables age, gender, smoking history, histology, tumour size, FEV1 and history of previous pulmonary resections were used as covariates. After estimation of the propensity score, patients were matched using a simple 1:1 nearest neighbour matching algorithm, imposing a caliper width of 0.1. No imbalances remained as assessed through univariate (χ2) and multivariate (L1) tests. All measures indicated that the propensity score matching improved the overall balance. The matched sample included a total of 280 patients, evenly distributed in the two groups (open segmentectomy versus VATS segmentectomy). There were no differences regarding any of the variables used as covariates in the matching process.
Results
Clinical characteristics and surgical procedures
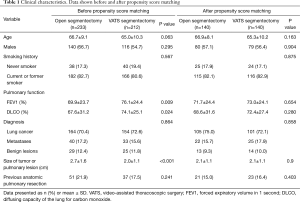
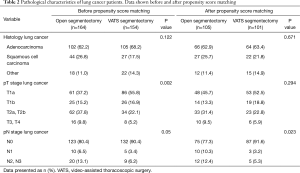
From January 2006 to December 2015, 233 patients (52.4%) underwent segmentectomy by open thoracotomy and 212 patients (47.6%) by VATS. The frequency distribution of both procedures performed per study year is depicted in Figure 1. Clinical characteristics and the pathological data of the lung cancer patients are shown in Tables 1,2, respectively.

Full table
Full table
There were no differences in age, gender, smoking history, diagnosis, histology of lung cancer and history of previous pulmonary resections. However, patients in the open segmentectomy group had lower preoperative FEV1 (P=0.009) and DLCO values (P=0.024) as well as a larger tumor size (P<0.001).
In patients with primary lung cancer, the most frequent histologic subtypes were adenocarcinoma (207 cases; 61.2%) and squamous cell carcinoma (71 cases; 21%). The most common primary tumors in patients with pulmonary metastases were colorectal carcinoma (28 cases; 38.4%), transitional cell carcinoma in (9 cases; 12.3%), breast cancer (8 cases; 11%) and lung cancer (7 cases; 9.6%). The most frequent benign lesions were pulmonary infiltrates (11 cases; 20.4%), bronchiectasis (9 cases; 16.7%) and localized fibrosis (8 cases; 14.8%).
A total of 69 (21.7%) patients treated for lung cancer and 15 patients (20.5%) treated for metastases had a surgical history of previous anatomic pulmonary resection (lobectomy or segmentectomy). This proportion was smaller in the group of patients with benign lesions where 4 patients (7.4%) underwent lung resection before. Prior thoracic operations were due to lung cancer (83%), metastases (13.6%) and benign lesions (3.4%).
After propensity score matching, the two study groups were comparable with respect to age, gender, smoking history, diagnosis, tumour size, FEV1, DLCO and history of previous pulmonary resections (Table 1). There were no differences between the two groups regarding histology and pT stage in lung cancer, however, lymph nodes were more frequently affected in the open segmentectomy group (P=0.023) (Table 2).
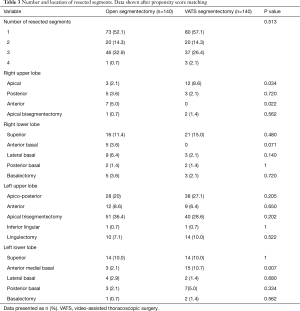
Segmentectomy locations and the number of resected segments are shown in Table 3. In 153 patients (54.6%) resection was limited to a single segment, whereas in 127 patients (45.4%) multiple segments were resected. In 122 of cases with multiple resections (96.1%) neighboring segments were resected, whereas five patients (3.9%) received a resection of two or more independent segments. A total of 92 patients (32.9%) underwent right-sided pulmonary resection, whereas 185 (66.1%) were resected on the left side. Three patients (1.1%) received bilateral resections, two of whom were due to lung cancer and one to metastatic disease. On the right side, the superior segment of the lower lobe was the most common segmental resection performed (n=37; 13.2%) followed by the apical segment of the upper lobe (n=15; 5.4%). The most frequent left-sided segmental resections were the upper lobe trisegmentectomy (n=73; 26.1%) and the apical posterior segmentectomy of the upper lobe (n=66; 23.6%). A lingulectomy was performed in 28 patients (8.6%).
Full table
Postoperative outcomes
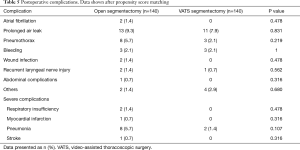
Table 4 shows the postoperative outcomes. There were no significant differences in operative time, radicality of resection and frequency of lymphadenectomy. Patients in the open group spent more time in hospital (9.5 vs. 7.4 days, P<0.001) and had a longer duration of thoracic drainage treatment (5.9 vs. 4.7 days, P=0.012) compared to patients in the VATS group. In 56 patients (20.0%) postoperative complications were reported (Table 5). The most frequent complications were prolonged air leak (24 cases; 8.6%), pneumothorax (11 cases; 3.9%), pneumonia (10 cases; 3.6%) and postoperative bleeding (6 cases; 2.1%). Patients suffering complications had a prolonged hospital length of stay (12.2 vs. 7.5 days, P<0.001) and drainage treatment time (9.0 vs. 4.4 days, P<0.001) compared to patients with an uncomplicated postoperative course. Overall complications rates were similar in both groups (open 23.6%, VATS 16.4%, P=0.135). However, severe complications were more frequent in the open group (7.1%) than in the VATS group (1.4%, P=0.018).

Full table
Full table
There were no postoperative deaths.
Discussion
In the current study, we compare the perioperative outcome of patients receiving anatomic segmentectomy, either by open or video-assisted thoracoscopic approach. Perioperatively there were no significant differences in operative time, frequency of lymphadenectomy or radicality. The main findings of the present study were that VATS segmentectomy was associated with a decreased hospital length of stay and decreased incidences of severe complications compared with open segmentectomy.
The technique of segmentectomy was first described by Churchill and Belsey in 1939 for the treatment of bronchiectasis (in this case a lingulectomy). As stated in the article, the substantial advantage of this procedure is the preservation of healthy lung tissue, which allows the resection of all diseased segments from several lobes. Until today, the conservation of functional lung tissue is the central argument for segmentectomy. Being the smallest possible anatomic resection, it allows lung cancer patients with poor pulmonary reserve to undergo a curative resection. Several studies could demonstrate that segmental resection offers a better functional preservation compared to lobectomy (18-20). However, the difference in preserved pulmonary function narrows with an increasing number of resected segments. In a comparative study of the postoperative pulmonary function after segmentectomy versus lobectomy in 140 patients, resection of the left upper division did not differ from left upper lobectomy in preserving pulmonary function (18). In our study, multiple segments were resected in 45.4% of the patients. In most of these cases (96.1%), neighboring segments were resected for the treatment of solitary pulmonary lesions, whereas a small proportion (3.9%) of patients received resection of independent segments. Factors influencing the extent of resection, especially in malignant lesions, are the tumor size including adequate resection margins as well as its location within the lung. In contrast to small peripheral tumors that can be clearly allocated to a certain segment, larger or centrally located tumors can cross the segmental boundaries and therefore require a more extensive resection. Another reason for the frequent resection of multiple segments might be deficits in preoperative planning by computed-tomography (CT). Even in high resolution imaging a clear allocation of the pulmonary lesion to a certain segment remains difficult and decisions concerning the extent of resection are often made intraoperatively. Advanced 3D-imaging techniques that allow the reconstruction of the corresponding segmental vessels and bronchus could facilitate preoperative planning and therefore reduce the extent of resection in the future.
Regarding the influence of the operative technique (open versus thoracoscopic surgery) on the perioperative outcome, most studies focused on early stage lung cancer. Limited data are currently available assessing the potential advantages of thoracoscopic segmentectomy for more advanced disease as well as for the treatment of pulmonary metastases and benign lesions. In 2007 Atkins and colleagues conducted a retrospective analysis on 77 consecutive segmentectomies for the treatment of malignant and benign pulmonary lesions (15). In their study, 48 patients underwent VATS and 29 open surgeries. Primary tumors were significantly smaller in the VATS group than in the thoracotomy group (2.1 vs. 3.1 cm). In addition, 95% of the patients in the VATS group treated for lung cancer were stage I, whereas 25% of patients in the thoracotomy group had more advanced disease (stage IIB and higher). There were no differences in operative time, blood loss and chest tube duration. However, the hospital length of stay was significantly shorter in the VATS group (4.3 vs. 6.8 days). There were no significant differences regarding overall morbidity (34.5% vs. 31.3%) and thirty-day mortality (0% vs. 6.9%) between the two groups (15). In 2009, Schuchert and colleagues performed a retrospective review of patients that underwent segmentectomy for stage I non-small cell lung cancer, either by VATS (n=104) or thoracotomy (n=121) (14). The tumor size was slightly higher in the open group compared with the VATS group (2.4 vs. 2.1 cm). Patients in the VATS group had a shorter length of stay (5 vs. 7 days) than patients in the open group. The overall postoperative morbidity was 30.2% with no differences between the two groups. There rate of pulmonary complications (25% vs. 40%) and postoperative infections (2.9% vs. 10.7%) were significantly lower in the VATS group (14). In 2014, Smith and colleagues compared the postoperative outcomes of elderly stage I lung cancer patients treated with segmentectomy via VATS versus an open approach. After adjusting for propensity scores baseline characteristics were not different between the groups. Postoperatively both groups showed similar rates of postoperative complications (39.2%), extended length of stay (15.9%) and postoperative mortality (3.1%) (17). In our study, which included cases with more advanced disease (T >2a, N+) as well as patients with lung metastases and benign pulmonary lesions, the overall morbidity observed was 20.0%, with no postoperative deaths, which is comparable to data reported in the literature (14,16,21-23).
Segmentectomy has been suggested as a reasonable compromise for patients that would not tolerate lobectomy because of limited cardiopulmonary reserves, for example in cases of previous pulmonary resections (14,24,25). In the present study, 19.8% of the patients had undergone a prior anatomic pulmonary resection. This was also the limiting factor in many of our cases with tumors exceeding two centimeters in diameter or in patients who had primarily a R1/R2 resection. Although accurate figures are not available concerning the rate of reoperations in lung surgery, nearly 20 percent appears to be a considerably high proportion. In 1996, Cerfolio and associates conducted a retrospective analysis of 83 patients with compromised pulmonary function (FEV <1.2 L) who underwent resection for lung cancer (26). In this study 11 patients (13%) have had a thoracotomy before, 9 for lung cancer and 2 for post-traumatic bleeding. Yang and D’Amico showed that 11.5% of patients undergoing surgery for lung cancer develop additional primary lung cancer within their lifetimes and may require further lung tissue resection. They concluded that segmentectomy, which preserves more lung function than lobectomy, would offer higher tolerance for further resections (3).
Although the data in our study were acquired prospectively, the patient collective was analyzed retrospectively, which must be regarded as a limitation. In the study period of seven years, operative techniques and indications concerning feasibility of VATS segmentectomy evolved all over the world, including our institution. Primary indication for open segmentectomy was re-do anatomical resection on the pre-operated side. Severe hilar scare tissue was the major concern in this patients and therefore open technique was primarily indicated. Nevertheless, improving thoracoscopic techniques allow to resect prior operated patients minimally invasive without major intraoperative complications, making today open segmentectomy a rare operation in our institution (Figure 1).
A second limitation is the relatively high proportion of patients with more advanced disease in our study. In total, 35% of the patients in the matched sample had a T2a stage and above. According to the actual recommendations, this would have been patients normally scheduled for lobectomy. The individual reasons for choosing segmentectomy in these patients were not recorded in this study. Reasonable explanations for performing a sublobar resection could be the poor pulmonary function and the high proportion of previous pulmonary resections in our study. Although segmentectomy is not considered equivalent in local control compared to lobar resection, in patients not suitable for lobectomy, however, it might be the best chance of curative resection.
In our study 9% of the patients exhibited a N2/3 disease despite preoperative staging. Although N+ disease is an independent factor for perioperative morbidity and mortality, we did not exclude those patients from our statistical analysis. The aim of our study was to represent clinical reality and therefore assess the perioperative outcome of the procedure itself, regardless of histology, tumor entity and stage.
In conclusion, our data suggest that VATS segmentectomy is a safe and effective technique for the treatment of benign and malignant pulmonary lesions. We demonstrated significant advantages of the thoracoscopic approach, such as shorter hospitalization time and a decreased incidence of severe complications. Minimizing the amount of lung tissue resected, combined with the minimally invasive approach, make the VATS segmentectomy highly suitable for patients with reduced pulmonary function or severe comorbidities.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the Institutional Review Board of the Hospital of the City of Cologne, Germany (No. 126/2016). Informed consent was obtained from all patients.
References
- Vansteenkiste J, Crino L, Dooms C, et al. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol 2014;25:1462-74. [Crossref] [PubMed]
- Detterbeck FC, Mazzone PJ, Naidich DP, et al. Screening for lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e78S-92S.
- Yang CF, D'Amico TA. Thoracoscopic segmentectomy for lung cancer. Ann Thorac Surg 2012;94:668-81. [Crossref] [PubMed]
- Okada M, Nishio W, Sakamoto T, et al. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg 2005;129:87-93. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:926-32; discussion 32-3. [Crossref] [PubMed]
- Yang CF, D'Amico TA. Open, thoracoscopic and robotic segmentectomy for lung cancer. Ann Cardiothorac Surg 2014;3:142-52. [PubMed]
- Berry MF. Role of segmentectomy for pulmonary metastases. Ann Cardiothorac Surg 2014;3:176-82. [PubMed]
- Mitchell JD, Yu JA, Bishop A, et al. Thoracoscopic lobectomy and segmentectomy for infectious lung disease. Ann Thorac Surg 2012;93:1033-9; discussion 9-40. [Crossref] [PubMed]
- Churchill ED, Belsey R. Segmental Pneumonectomy in Bronchiectasis: The Lingula Segment of the Left Upper Lobe. Ann Surg 1939;109:481-99. [Crossref] [PubMed]
- Paul S, Isaacs AJ, Treasure T, et al. Long term survival with thoracoscopic versus open lobectomy: propensity matched comparative analysis using SEER-Medicare database. BMJ 2014;349:g5575. [Crossref] [PubMed]
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 5-6. [Crossref] [PubMed]
- Schuchert MJ, Pettiford BL, Pennathur A, et al. Anatomic segmentectomy for stage I non-small-cell lung cancer: comparison of video-assisted thoracic surgery versus open approach. J Thorac Cardiovasc Surg 2009;138:1318-25.e1. [Crossref] [PubMed]
- Atkins BZ, Harpole DH Jr, Mangum JH, et al. Pulmonary segmentectomy by thoracotomy or thoracoscopy: reduced hospital length of stay with a minimally-invasive approach. Ann Thorac Surg 2007;84:1107-12; discussion 12-3. [Crossref] [PubMed]
- Leshnower BG, Miller DL, Fernandez FG, et al. Video-assisted thoracoscopic surgery segmentectomy: a safe and effective procedure. Ann Thorac Surg 2010;89:1571-6. [Crossref] [PubMed]
- Smith CB, Kale M, Mhango G, et al. Comparative outcomes of elderly stage I lung cancer patients treated with segmentectomy via video-assisted thoracoscopic surgery versus open resection. J Thorac Oncol 2014;9:383-9. [Crossref] [PubMed]
- Nomori H, Cong Y, Sugimura H. Systemic and regional pulmonary function after segmentectomy. J Thorac Cardiovasc Surg 2016;152:747-53. [Crossref] [PubMed]
- Saito H, Nakagawa T, Ito M, et al. Pulmonary function after lobectomy versus segmentectomy in patients with stage I non-small cell lung cancer. World J Surg 2014;38:2025-31. [Crossref] [PubMed]
- Takizawa T, Haga M, Yagi N, et al. Pulmonary function after segmentectomy for small peripheral carcinoma of the lung. J Thorac Cardiovasc Surg 1999;118:536-41. [Crossref] [PubMed]
- Hwang Y, Kang CH, Kim HS, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy on the patients with non-small cell lung cancer: a propensity score matching study. Eur J Cardiothorac Surg 2015;48:273-8. [Crossref] [PubMed]
- Gossot D, Zaimi R, Fournel L, et al. Totally thoracoscopic pulmonary anatomic segmentectomies: technical considerations. J Thorac Dis 2013;5 Suppl 3:S200-6. [PubMed]
- Fournel L, Zaimi R, Grigoroiu M, et al. Totally thoracoscopic major pulmonary resections: an analysis of perioperative complications. Ann Thorac Surg 2014;97:419-24. [Crossref] [PubMed]
- Martin-Ucar AE, Delgado Roel M. Indication for VATS sublobar resections in early lung cancer. J Thorac Dis 2013;5 Suppl 3:S194-9. [PubMed]
- Lau KK, Martin-Ucar AE, Nakas A, et al. Lung cancer surgery in the breathless patient--the benefits of avoiding the gold standard. Eur J Cardiothorac Surg 2010;38:6-13. [Crossref] [PubMed]
- Cerfolio RJ, Allen MS, Trastek VF, et al. Lung resection in patients with compromised pulmonary function. Ann Thorac Surg 1996;62:348-51. [Crossref] [PubMed]


