Indwelling pleural catheters: complications and management strategies
Introduction
Recurrent pleural effusions (RPEs) are defined as pleural effusions that recur despite optimal therapy for the underlying etiology of the effusions and typically require multiple thoracenteses or a more definitive therapeutic modality to prevent recurrence. Indwelling pleural catheters (IPCs) have emerged in the past decade as a very effective modality to treat and control RPEs (1). They have also been shown to be associated with fewer hospitalization days from treatment to death when compared to talc pleurodesis (2).
Warren et al. compared IPCs to chest tubes and chemical pleurodesis and concluded that chest tubes and chemical pleurodesis had a 30% higher effusion recurrence rate than IPCs (3). Additional advantages of IPCs include a low failure rate, improved quality of life, lower initial cost, and a shorter initial admission time (4). The procedure is usually done on an ambulatory basis under a local anesthetic. Treating RPEs with IPCs however, doesn’t come without complications. These complications could occur early (procedure-related complications) (5) or months after placement (catheter-related complications) (6). As more patients are being treated with IPCs for benign as well as malignant pleural effusions, physicians need to be aware of how to identify and treat these catheter-related complications.
The purpose of this review is to discuss the complications of IPCs, their recognition, management, and how they can be potentially avoided.
Procedure-related complications
Procedure-related complications are usually acute and similar in frequency to those observed with any other pleural intervention. These include pneumothorax, subcutaneous emphysema, bleeding, and overlying skin infections. Pneumothorax is seen more commonly in patients with trapped lung (5). The early complications are reported to occur at a rate of 2.8% to 6% (1,7). Procedure-related complications are usually managed in a manner similar to when they occur during any other pleural intervention and will not be discussed in this review.
Catheter-related complications
Pleural infection
Catheter-related pleural infections (CRPIs) are usually mild and the majority of cases are controlled with appropriate antibiotic therapy alone without the need to remove the catheter (6). When a patient with IPC develops signs of infection and the drainage becomes purulent or the gram stain or cultures are positive for a specific organism, the diagnosis of CRPI is straightforward. In some instances, however, the diagnosis might be challenging, especially in malignant pleural effusions since these effusions are known to cause low glucose and high lactate dehydrogenase (LDH) levels (6). Factors that help to establish a diagnosis include a change in the pleural fluid cell count and differential and an increase in the pleural fluid LDH with a drop in the pleural fluid glucose levels compared to the initial fluid analysis. The pleural fluid cell count increases and becomes neutrophilic (8). Fysh et al. reported their experience with CRPIs in 11 centers in North America. Out of a total of 1,021 patients, 4.9% developed a CRPI. Ninety-four percent of patients with CRPIs were successfully treated with antibiotic therapy. Sixty-two percent of subjects required at least one dose of intravenous antibiotic therapy. Thirty-seven out of 50 patients (74%) were hospitalized. The median duration of antimicrobial therapy was 24 [interquartile range (IQR), 14–42] days; 38% of patients were treated solely with oral antibiotics, and the remainder received at least one intravenous dose. No difference in complete resolution rate was found between those treated with intravenous versus oral antibiotics. No patient needed surgery for treatment of the pleural infection (9).
CRPIs can be categorized into three different forms
Cellulitis
Cellulitis is usually treated with outpatient oral antibiotic therapy. Faiz et al. described their single center experience with IPCs in patients with hematologic malignancies. The time to local infections (cellulitis and exit site infections) among their patients ranged from 1 to 6 weeks, and they were typically managed in an outpatient setting with oral antimicrobial therapy (10). Similarly, Skalski et al. reported their experience in a transplant and non-transplant cohort where cellulitis was successfully treated with oral antibiotics (11).
Empyema
Empyema or pleural fluid infection is usually treated with continuous IPC drainage with antibiotic therapy. Some authors have reported on the use of tissue plasminogen activator (tPA) and deoxyribonuclease (DNase) in addition to antibiotic therapy in patients with IPC and empyema (9). In most cases intravenous antibiotics is required. When antibiotic therapy alone fails, treatment is usually similar to treating empyema. The IPC should be removed and a chest tube is placed. Sometimes, a more aggressive surgical intervention might be needed to eradicate the infection. These aggressive measures are usually reserved for subjects with good functional status and a life expectancy of more than few months. The mortality rate from CRPI is 0.29% (9). Staphylococcus aureus is the most common causative agent and is found in about 50% of cases. Other etiologic agents include Pseudomonas Aeruginosa and Enterobacteriaceae (10). The mechanism of infection has been proposed to be from entry of bacteria through and around the catheter. In certain circumstances, a pneumonic process may be responsible. Skalski et al. described their experience with solid organ transplant and non-transplant patients. Out of 19 patients there were two empyemas. Both patients required hospitalization and intravenous antibiotics. The first empyema occurred 79 days and the second 53 days after the IPC placement. In both cases the IPC was left in place and both patients experienced pleurodesis after recovery from the infection (11). In another instance, Abrão et al. reported one case of empyema which was treated with antibiotics while the IPC was left in place until the infection resolved (12).
Faiz et al. reported five cases of empyemas in a population of patients who received IPCs for pleural effusions secondary to hematologic malignancies. All cases were associated with a bacterial pathogen (methicillin-resistant Staphylococcus aureus, coagulase-negative S. aureus) and all patients required hospitalization. One patient had video-assisted thoracoscopic surgery (VATS) with decortication (10). Fysh et al. reported that the majority of pleural infections (54.0%, n=27) were successfully managed without removing the IPC. Of the 23 IPCs removed in an attempt to assist infection control, 11 (47.8%) required another IPC or another form of drainage for recurrent malignant effusion. Intrapleural fibrinolytics were administered via IPC in 13 (26.0%) patients: Six received combination therapy of tPA and DNase, four had streptokinase, two had tPA alone, and one had urokinase. Eleven out of these 13 (84.6%) patients had complete resolution of their infection and the remaining two patients (one with tPA and one tPA plus DNase) had chronic infections requiring long-term antibiotics (9).
In the quality improvement project by Gilbert et al., a protocol was established to overcome the high IPC-related infection rate at their institution (8.2%). Interventions implemented included strict adherence to a sterile protocol, a single hospital site to perform the IPC placement (the endoscopy suite in the study). All patients received perioperative antibiotics (cefazolin 1 g intravenously; if allergic to cefazolin, then vancomycin 1 g intravenous was administered) within 60 minutes prior to IPC insertion (13). IPC-related infections were significantly reduced from 8.2% to 2.2% (P=0.0049). The relative risk reduction was 73%. Until further data becomes available, however, the authors at this time cannot recommend peri-insertion antibiotic prophylaxis. The infection rate for benign pleural effusions (BPE) has been reported to be less than that for MPE. Patil et al. reported a 2.3% rate of empyema in BPE (14). Table 1 compares IPC complications rate between benign and malignant pleural effusions.
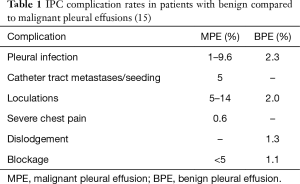
Full table
Drug eluting intrapleural catheters are currently under investigation to assess their effectiveness in creating pleurodesis. Silver nitrate coated catheters have been used in animal experimental models. A study by Tremblay et al. showed improved pleurodesis scores in both lamb and rabbit models after use of silver nitrate coated catheters for pleurodesis. This approach could potentially be useful in achieving pleurodesis on an outpatient basis for patients with a variety of pleural diseases (16,17).
Tunnel infections
Tunnel infections are defined as erythema, tenderness, and induration overlying the tunnel tract and extending more than 2 cm from the catheter exit site. Most of these infections are managed with antibiotics. In Faiz’s report on IPCs in patients with hematological malignancies, 172 patients experienced complications. Three of these complications were related to tunnel site infections, all of which were managed with antibiotics therapy alone (10).
Catheter tract metastases/seeding
Catheter tract metastases are uncommon, occurring in less than 5% of cases. Over half of the cases in reported studies have been associated with mesothelioma (18). Sartori et al. reported a case of a 72-year-old male with an IPC inserted for malignant RPE secondary to mesothelioma. The subject received intra-pleural chemotherapy with subsequent clearance of the effusion and the IPC was removed a week later. Three months later, the patient was diagnosed with biopsy-proven subcutaneous metastasis of mesothelioma (19). Subcutaneous metastasis has also been reported in a patient with abdominal mesothelioma who received a peritoneovenous shunt and in another case of pleural mesothelioma with a Port-A-Cath in place (20).
The seeding is hypothesized to occur from migration of cells along the subcutaneous tract of the catheter. In the case of pleural catheter, the seeding can be caused from a higher intra-pleural pressure causing a leakage of fluid after initial insertion. This may potentially be avoided by removing the pleural fluid via thoracentesis prior to placement of an indwelling catheter (21). Most metastases occur several weeks after catheter insertion supporting the mechanism of tumor cell migration and subsequent growth leading to clinical detection. Management may include radiation therapy for isolated lesions if no other site of systemic progression exists. Prophylactic radiotherapy has been reported as a preventive measure for subcutaneous seeding but existing data is insufficient to warrant its routine use (22).
Loculations
Symptomatic loculations occur in up to 14% of patients with IPCs (23). The etiology is related to the accumulation of fibrinous material that forms septations leading to multiple loculations. These septations and loculations lead to impaired fluid removal causing fluid accumulation that causes dyspnea and discomfort (24). Thomas et al. described the use of intrapleural fibrinolytic therapy in 66 patients with symptomatic loculations after IPC placement. The fibrinolytic agents used were tPA, Streptokinase, or Urokinase. Following treatment, 93% of patients had improvement in their pleural fluid drainage (defined as an increase in pleural fluid drainage by 500 mL) and 83% of patients had relief of their dyspnea. Two patients had significant pleural bleeding (3%). One patient had solid pleural metastases from renal cell carcinoma and developed a symptomatic hemoglobin drop of 4.8 g/dL 2 days after fibrinolytic treatment. The other patient had metastatic breast carcinoma and developed a significant fall in hemoglobin of 6 g/dL 3 days after therapy. Both patients remained hemodynamically stable and responded to supportive management along with packed red blood cells (RBC) transfusions. Neither case required invasive intervention. Symptomatic loculations recurred in 27 (40.9%) patients after 2 to 69 days (median, 13 days). Of these, 10 received repeat fibrinolytic therapy and three patients had a second IPC inserted. Only one patient had a sustained improvement in drainage and symptoms following the second dose of fibrinolytic therapy (25). Figure 1 shows an example of pleural loculations treated successfully with intrapleural tPA.
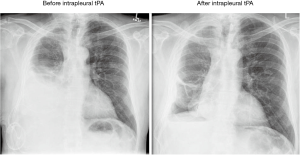
Other reported treatment modalities for symptomatic loculations include intra-pleural DNase or a combination of DNase and tPA (26). Even though, these measures may be temporary and short-lasting, they do provide significant relief of symptoms which is the primary purpose of IPC particularly in the setting of advanced malignancy.
VATS or medical pleuroscopy is not used very commonly as a therapeutic option for loculations. Raman et al. reported a single center experience for management of RPEs with tunneled pleural catheters (TPCs). One hundred and ninety-three TPCs were placed, alteplase was used in seven patients to resolve unsatisfactory loculations or catheter obstructions to improve drainage. Two patients developed hemothoraces and had to have a thoracotomy to evacuate the pleural cavity. Six patients required repeat medical pleuroscopy and one required (VATS). Of the six patients who had a repeat pleuroscopy, three were patients who were being treated for breast cancer and were diagnosed with adenocarcinoma of the lung on the second procedure. VATS was performed for one patient in whom the pleural biopsies were unable to confirm a diagnosis of mesothelioma and he eventually required a pleurectomy to establish the diagnosis (10).
Chest pain
Chest pain is frequently encountered after IPC placement. It occurs in 36% of patients but is usually mild and resolves within 3 days after insertion (27). This pain can be usually easily managed with analgesics. Negative pressure that develops during drainage of the IPC may result in pain. This type of pain occurs more commonly and is usually more intense when the IPC is placed in the setting of trapped lung. This is managed by slowing or stopping the drainage. Fortunately, severe intractable pain occurs less often, only in 0.6% of cases. This pain is usually severe and refractory to pain medications (5). It only responds to catheter removal. Some cases reported on radiation therapy for IPC related chest pain but with conflicting results (28).
Immunosuppression and malnutrition from chronic pleural fluid drainage
Persistent long-term drainage of pleural fluid can result in significant nutritional material and cellular losses. It is estimated that one liter of exudative pleural fluid contains up to 30 grams of protein (29). Patients with malignant pleural effusions may already be immunosuppressed, cachectic, and malnourished and they may be unable to compensate for the extra nutritional losses that occur from draining their pleural fluid on a regular basis. Jimenez et al. reported a decline in serum albumin level in patients with IPCs placed for malignant chylothoraces (30). The albumin level recovered after removal of the catheter. On the other hand, Fysh et al. compared talc pleurodesis to IPC. No significant difference in the rate of protein or albumin loss was seen between the two groups (9). In studies that looked at electrolyte imbalances after IPC placement, there were no significant electrolyte abnormalities reported in patients with end-stage renal disease, heart failure, hepatic hydrothorax, or chylothorax (14,15,31,32).
At the present time, immunosuppression and nutritional losses from chronic pleural drainage remain a theoretical risk. The significant benefit that IPCs offer patients, especially those with malignant pleural effusions, outweighs the potential risk of immunosuppression or nutritional losses.
Dislodgement
The rate of dislodgement after IPC placement is variable. Tremblay reported a rate of 0.9% (1) whereas van den Toorn et al. reported a rate as high as 18% (33). The majority of patients who had their IPC dislodged where patients with malignant pleural effusions or were patients on chemotherapy (1). A possible explanation for this predilection could be related to immunosuppression and cancer-associated cachexia that could delay catheter adherence to the site of entry. One simple solution to this possible complication in this patient subgroup (patients on chemotherapy or those with cancer-associated cachexia) is to keep the anchoring suture at the entry site of the catheter for a longer duration.
Blockage
Catheter blockage is an uncommon complication and occurs in less than 5% of cases. Partial blockage is generally more common than complete blockage (9). Blockage of IPCs can result from accumulation of fibrinous exudates inside as well as around the catheter lumen. This complication can be usually managed by flushing the catheter with saline solution using aseptic technique. The fibrinolytic agent alteplase has been used to relieve obstruction of blocked catheters. Failure of saline flush to restore patency of the catheter is usually followed by instillation of a fibrinolytic agent to restore drainage of the intrapleural catheter. A study of 37 patients with non-draining IPCs found a high success rate for restoration of flow after tPA instillation with no associated complications (34). In addition, in a study by Vial et al., restoration of flow in patients treated with one dose of tPA was seen in 83 of 97 patients [86%; 95% confidence interval (CI), 77–92%] (35).
Catheter removal and replacement is sometimes necessary for those completely blocked catheters that did not respond to saline flushing or tPA instillation. Some of these patients eventually require further invasive procedures like repeat thoracentesis, IPC or chest tube placement (9).
Peri-catheter leakage
Peri-catheter leakage has been reported in up to 13% of patients, it is usually self-limited and rarely requires surgical intervention (36). The mechanism is hypothesized to be similar to that for catheter tract metastases whereby following insertion of the IPC, the high intra-pleural pressure causes leakage of pleural fluid around the IPC with drainage to the skin surface. This fluid can potentially be irritating to the skin. This complication can be avoided by prior drainage of the pleural fluid and firmly securing the catheter to the site of placement (36).
Catheter fracture during removal
Fracture of an IPC catheter usually occurs at the time of removal. Catheter removal may be indicated in cases of spontaneous pleurodesis and resolution of the pleural effusion or a serious complication such as empyema or intractable pain. Breakage can occur while attempting to release the cuff from the surrounding tissue. When the catheter has been in place for a longer period, adhesions may form making it difficult to remove the catheter without inadvertently breaking it. In a case series reported by Fysh et al., 61 of the 171 IPC’s placed were removed. Among those, 9.8% were severed. They described six cases of retained IPCs where fragments of one IPC remained in place for a duration of 1,119 days without any complication (37). Other patients with retained fragments did not develop any further complications either. In summary, physicians should be aware that IPCs can fracture during their removal. If fracture occurs, the retained fragment should be left in place without aggressive retrieval measures (i.e., thoracoscopy) (37). Two factors that could lead to a low catheter fracture rate include, first placing the cuff at a short distance from the entry site, and second to remove the catheter as soon as pleurodesis occurs. Table 2 summarizes the complications related to IPCs and how to manage them.

Full table
Complications in specific patient populations
Two patient populations deserve special mention: patients with hematologic malignancies, and solid organ transplant recipients.
Hematologic malignancies
In patients with hematologic malignancies there is at least, a theoretical concern about the risk of infection with long term indwelling catheters. Patients with hematologic malignancies generally have suppressed immune systems, and after chemotherapy, patients are frequently neutropenic which further impede their ability to fight infections. Published data, however, have not shown an increased risk of infection in patients with hematologic malignancies who received an IPC (38). Gilbert et al. reported on 91 patients with hematologic malignancy who received an IPC. CRPIs occurred in 7 (7.7%) patients, a rate that is similar to CRPIs in patients who received an IPC for other indications (39). Another potential concern in patients with hematologic malignancies receiving an IPC is the risk of bleeding (40). Most patients with hematologic malignancies have low platelet counts and could have abnormal bleeding diathesis. In a large meta-analysis, the reported bleeding risk in patients with hematologic malignancies was higher compared to the control group (1.7% compared to 0.4%) (41).
Solid organ transplant
Patients with solid organ transplant are on immunosuppressive therapy and are usually considered at increased risk of infections. This raises the concern of CRPI in this patient’s population. In a recent case series, the rate of CRPI in a group of 19 patients with solid organ transplant who received an IPC was similar to a control group of 55 patients (16.9% compared to 11%) (42,43).
Conclusions
As the use of IPCs is becoming widespread, more patients and physicians will possibly be faced with increasing likelihood of complications related to the long-term presence of a pleural catheter. Most of these complications are usually managed conservatively. Some complications, however, require catheter removal, and sometimes more aggressive surgical interventions. To date, evidence-based data on how to manage IPC related complications is scarce. More studies are needed to guide physicians and health care providers on how to avoid, recognize, and effectively treat IPC-related complications. Regardless of all potential complications, IPC remains the best modality to control recurrent malignant as well as non-malignant pleural effusions. It provides a very effective therapeutic modality on an outpatient basis with a seemingly low complication rate.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest 2006;129:362-8. [Crossref] [PubMed]
- Thomas R, Fysh ET, Smith NA, et al. Effect of an Indwelling Pleural Catheter vs Talc Pleurodesis on Hospitalization Days in Patients With Malignant Pleural Effusion: The AMPLE Randomized Clinical Trial. JAMA 2017;318:1903-12. [Crossref] [PubMed]
- Warren WH, Kalimi R, Khodadadian LM, et al. Management of Malignant Pleural Effusions Using the Pleurx Catheter. Ann Thorac Surg 2008;85:1049-55. [Crossref] [PubMed]
- Boshuizen RC, Thomas R, Lee YC. Advantages of indwelling pleural catheters for management of malignant pleural effusions. Curr Respir Care Rep 2013;2:93-9. [Crossref]
- Wrightson JM, Fysh E, Maskell NA, et al. Risk reduction in pleural procedures: sonography, simulation and supervision. Curr Opin Pulm Med 2010;16:340-50. [Crossref] [PubMed]
- Lui MM, Thomas R, Lee YC. Complications of indwelling pleural catheter use and their management. BMJ Open Respir Res 2016;3. [Crossref] [PubMed]
- Sioris T, Sihvo E, Salo J, et al. Long-term indwelling pleural catheter (PleurX) for malignant pleural effusion unsuitable for talc pleurodesis. Eur J Surg Oncol 2009;35:546-51. [Crossref] [PubMed]
- Hooper C, Lee YC, Maskell N, et al. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii4-17. [Crossref] [PubMed]
- Fysh ET, Tremblay A, Feller-Kopman D, et al. Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest 2013;144:1597-602. [Crossref] [PubMed]
- Faiz SA, Pathania P, Song J, et al. Indwelling Pleural Catheters for Patients with Hematologic Malignancies. A 14-Year, Single-Center Experience. Ann Am Thorac Soc 2017;14:976-85. [Crossref] [PubMed]
- Skalski JH, Pannu J, Sasieta HC, et al. Tunneled Indwelling Pleural Catheters for Refractory Pleural Effusions after Solid Organ Transplant. A Case-Control Study. Ann Am Thorac Soc 2016;13:1294-8. [Crossref] [PubMed]
- Abrão FC, Abreu B, Cavalcanti MG, et al. Use of indwelling pleural catheters for the definitive treatment of malignant pleural effusion. J Bras Pneumol 2017;43:14-7. [Crossref] [PubMed]
- Gilbert CR, Lee HJ, Akulian JA, et al. A Quality Improvement Intervention to Reduce Indwelling Tunneled Pleural Catheter Infection Rates. Ann Am Thorac Soc 2015;12:847-53. [Crossref] [PubMed]
- Patil M, Dhillon SS, Attwood K, et al. Management of Benign Pleural Effusions Using Indwelling Pleural Catheters: A Systematic Review and Meta-analysis. Chest 2017;151:626-35. [Crossref] [PubMed]
- Chalhoub M, Harris K, Castellano M, et al. The use of the PleurX catheter in the management of non-malignant pleural effusions. Chron Respir Dis 2011;8:185-91. [Crossref] [PubMed]
- Tremblay A, Dumitriu S, Stather DR, et al. Use of a drug eluting pleural catheter for pleurodesis. Exp Lung Res 2012;38:475-82. [Crossref] [PubMed]
- Tremblay A, Stather DR, MacEachern P, et al. Use of a Drug Eluting Pleural Catheter for Pleurodesis in a Large Animal Model. Am J Respir and Crit Care Med 2009;179:A4485.
- Lundstedt C, Stridbeck H, Anderson R, et al. A Tumor seeding occurring after fine-needle biopsy of abdominal malignancies. Acta Radiol 1991;32:518-20. [Crossref] [PubMed]
- Sartori S, Nielsen I, Trevisani L, et al. Subcutaneous seeding after ultrasound-guided placement of intrapleural catheter. An unusual complication of the intracavitary palliative treatment of pleural mesothelioma. Support Care Cancer 1999;7:362-4. [Crossref] [PubMed]
- van Ooijen B, Eggermont AM, Wiggers T. Subcutaneous tumor growth complicating the positioning of Denver shunt and intrapleural port-à-cath in mesothelioma patients. Eur J Surg Oncol 1992;18:638-40. [PubMed]
- Voravud N, Shin DM, Dekmezian RH, et al. Implantation metastasis of carcinoma after percutaneous fine-needle aspiration biopsy. Chest 1992;102:313-5. [Crossref] [PubMed]
- Boutin C, Rey F, Viallat JR. Prevention of malignant seeding after invasive diagnostic procedures in patients with pleural mesothelioma. A randomized trial of local radiotherapy. Chest 1995;108:754-8. [Crossref] [PubMed]
- Chee A, Tremblay A. The use of tunneled pleural catheters in the treatment of pleural effusions. Curr Opin Pulm Med 2011;17:237-41. [Crossref] [PubMed]
- Thomas R, Budgeon CA, Kuok YJ, et al. Catheter tract metastasis associated with indwelling pleural catheters. Chest 2014;146:557-62. [Crossref] [PubMed]
- Thomas R, Piccolo F, Miller D, et al. Intrapleural fibrinolysis for the treatment of indwelling pleural catheter-related symptomatic loculations: a multicenter observational study. Chest 2015;148:746-51. [Crossref] [PubMed]
- Pollak JS, Burdge CM, Rosenblatt M, et al. Treatment of malignant pleural effusions with tunneled long-term drainage catheters. J Vasc Interv Radiol 2001;12:201-8. [Crossref] [PubMed]
- Efthymiou CA, Masudi T, Thorpe JA, et al. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg 2009;9:961-4. [Crossref] [PubMed]
- Wrightson JM, Fysh E, Maskell NA, et al. Risk reduction in pleural procedures: sonography, simulation and supervision. Curr Opin Pulm Med 2010;16:340-50. [Crossref] [PubMed]
- Aapro M, Arends J, Bozzetti F, et al. Early recognition of malnutrition and cachexia in the cancer patient: a position paper of a European School of Oncology Task Force. Ann Oncol 2014;25:1492-9. [Crossref] [PubMed]
- Jimenez CA, Mhatre AD, Martinez CH, et al. Use of an indwelling pleural catheter for the management of recurrent chylothorax in patients with cancer. Chest 2007;132:1584-90. [Crossref] [PubMed]
- DePew ZS, Iqbal S, Mullon JJ, et al. The role for tunneled indwelling pleural catheters in patients with persistent benign chylothorax. Am J Med Sci 2013;346:349-52. [Crossref] [PubMed]
- Potechin R, Amjadi K, Srour N. Indwelling pleural catheters for pleural effusions associated with end-stage renal disease: a case series. Ther Adv Respir Dis 2015;9:22-7. [Crossref] [PubMed]
- van den Toorn LM, Schaap E, Surmont VF, et al. Management of recurrent malignant pleural effusions with a chronic indwelling pleural catheter. Lung Cancer 2005;50:123-7. [Crossref] [PubMed]
- Wilshire CL, Louie BE, Aye RW, et al. Safety and efficacy of fibrinolytic therapy in restoring function of an obstructed tunneled pleural catheter. Ann Am Thorac Soc 2015;12:1317-22. [Crossref] [PubMed]
- Vial MR, Ost DE, Eapen GA, et al. Intrapleural Fibrinolytic Therapy in Patients With Nondraining Indwelling Pleural Catheters. J Bronchology Interv Pulmonol 2016;23:98-105. [Crossref] [PubMed]
- Chalhoub M, Ali Z, Sasso L, et al. Experience with indwelling pleural catheters in the treatment of recurrent pleural effusions. Ther Adv Respir Dis 2016;10:566-72. [Crossref] [PubMed]
- Fysh ET, Wrightson JM, Lee YC, et al. Fractured indwelling pleural catheters. Chest 2012;141:1090-4. [Crossref] [PubMed]
- Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax 2011;66:448-9. [Crossref] [PubMed]
- Gilbert CR, Lee HJ, Skalski JH, et al. The use of indwelling tunneled pleural catheters for recurrent pleural effusions in patients with hematologic malignancies: a multicenter study. Chest 2015;148:752-8. [Crossref] [PubMed]
- Ibrahim U, Saqib A, Mohammad F, et al. KSHV-associated extracavitary primary effusion lymphoma in an HIV seronegative patient: a case report and review of the literature. Postgrad Med 2017;129:402-7. [Crossref] [PubMed]
- Mekhaiel E, Kashyap R, Mullon JJ, et al. Infections associated with tunneled indwelling pleural catheters in patients undergoing chemotherapy. J Bronchology Interv Pulmonol 2013;20:299-303. [Crossref] [PubMed]
- Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011;26:70-6. [Crossref] [PubMed]
- Schneider T, Reimer P, Storz K, et al. Recurrent pleural effusion: who benefits from a tunneled pleural catheter? Thorac Cardiovasc Surg 2009;57:42-6. [Crossref] [PubMed]


