Diffuse pulmonary meningothelial like nodules simulating metastatic thymoma
Introduction
Diffuse pulmonary meningotheliomatosis (DPM) is a parenchymal lung disease characterized by disseminated bilateral minute pulmonary meningothelial-like nodules (MPMNs). These meningothelial-like nodules are classically encountered as benign solitary or multiple pulmonary nodules containing small epithelioid cell collections nestled within normal pulmonary parenchyma.
In our clinical case, the patient presented with a newly diagnosed anterior mediastinal mass with associated multiple pulmonary nodules, which after atypical lung resection proved to be mesothelial like nodules. This is, to our knowledge, the first described case of MPMN simulating metastatic thymoma.
Case presentation
A 78-year-old woman was admitted to the Emergency Department of our Institution complaining of malaise, dyspnea, and inferior limbs pitting edema. Patient legs swelling have been increasing in the last 10 days.
At admission to Emergency Department she was conscious, Glasgow Coma Scale Score (GCSS) was 15, her blood pressure was 140/80 mmHg, SPO2 was 96% in room air, heart rate was 120 bpm.
History and clinical findings
Her past medical history included previous hysterectomy for fibromatosis, arterial hypertension, chronic atrial fibrillation diagnosed in 2000 for which she was put on regular anticoagulant therapy, chronic pericardial effusion diagnosed in 2009, hemodynamically stable, initially treated with medical therapy and subsequently with pericardiocentesis in 2016 for hemodynamic decompensation. Cytological analysis of the pericardial fluid was negative for tumor cells. She was under regular periodic echocardiographic follow-up every 6 months.
Preoperative investigation
The physical examination and laboratory findings were otherwise negative. Blood tests showed no abnormality including tumor markers such as a-fetoprotein, b-human chorionic gonadotropin, neuron-specific enolase and lactic acid dehydrogenase.
Electrocardiogram confirmed atrial fibrillation with normal ventricular rate. Chest X-ray showed increase in the cardiac shape in comparison to previous chest X-ray with cardiomegaly masking the inferior half of both lungs and blunting of both costophrenic diaphragmatic angles.
CT scan of the chest and abdomen revealed 55 mm × 50 mm, well-circumscribed solid and partially calcified mass in the anterior mediastinum (Figure 1). It also confirmed the presence of ubiquitous pericardial effusion, and showed some bilateral pulmonary micronodules, the largest of which localized in the right upper lobe, with maximal diameter of 7 mm. Moreover, the images reported bilateral pleural effusion, more pronounced on the left hemithorax. No abdominal lesions were found.

The patient underwent echographic-guided biopsy of the mediastinal mass. Pathological analysis of the mediastinal mass sample demonstrated type A thymoma.
Fluorodeoxyglucose-positron emission tomography (FDG-PET) highlighted pathological uptake at the level of the mediastinal mass only with a maximum standardized uptake value (SUVmax) of 8.4.
Because of the histological subtype with its usual benign behaviour the replicative nature of the pulmonary nodules was clinically not definitive. The case was discussed in a multidisciplinary setting and after evaluation and consensus the patient was scheduled for surgery.
Preoperative differential diagnosis included:
- Type A thymoma with multiple pulmonary metastases;
- Type A thymoma with associated occult metastatic second primary tumor;
- Type A thymoma with associated pulmonary nodules of inflammatory nature.
Surgery
In February 2016 the patient underwent open resection of the mediastinal mass, atypical pulmonary resection and pleuropericardial window were performed at one time to allow drainage of the pericardial fluid into the pleural space, characterize the pulmonary nodule through histopathological studies, and resect the mediastinal lesion.
Histology
The resected specimen consisted in right lower lobe wedge resection with small nodules ranging from 3 to 8 mm.
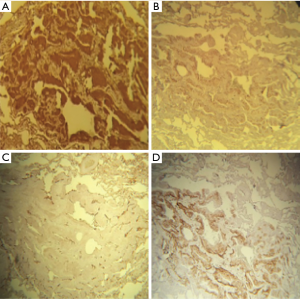
Histopathological examination showed some minute, nodular well-circumscribed lesions consisting of solid sheets of polygonal to oval cells with distinct cellular borders. The cytoplasm was clear to pale eosinophilic. The nuclei were round to oval, centrally located, with fine chromatin and inconspicuous nucleolus. No mitotic figures were seen (Figure 2).

Immunohistochemical analysis showed strong expression of epithelial membrane antigen (EMA), vimentin, CD56, and progesterone (Figure 3). Cytokeratin-cocktail and neuroendocrine markers (synaptophysin and chromogranin) were negative.
Outcome
Postoperative course was complicated by acute respiratory failure, which required intubation and transfer to the surgical intensive care unit.
She was then transferred back to the general floor and discharged from the hospital with fill recovery on postoperative day 12.
At 12 month’s follow up after surgery there were no evidence of local recurrence or metastasis has been detected in CT chest with contrast.
Literature revision and discussion
In 1960, Korn et al. (1) first described pulmonary meningothelial nodules as “pulmonary tumors resembling chemodectomas”, stemming from the belief that these nodules originated from oxygen-monitoring chemoreceptors due to geographic predominance near vascular structures. Three years later, the first cases of diffusely distributed or disseminated nodules of this type were described and this presentation was called pulmonary chemodectomatosis (2).
Subsequently, immunohistochemical and ultrastructural analysis discredited the original theory of chemoreceptor origin, as these structures were shown to resemble meningothelial cells (3). Gaffey et al. (4) in 1988 performed extensive immunohistochemical analysis of these nodules finding immunoreactivity for EMA and vimentin, two markers described in meningiomas (both intra and extracranial) and lack of reactivity to S-100 protein. Basing on these results, meningothelial origin for these structures were strongly advocated and the nomenclature: “minute pulmonary meningothelial nodules (MPMNs)” was coined, and first adopted in the third edition of the World Health Organization classification of tumors (5). Another important finding was immunoreactivity for progesterone receptor (PR).
However, the exact etiology of MPMNs remains unclear. MPMNs have been described in few case reports in literature and their nature and clinical significance have been discussed in some recent reviews (6).
The incidence of MPMNs varies in different reports according to the methods used to obtain pathological confirmation and on patient selection, ranging from 0.07–4.9% in autoptic series to 13.8% in more recent surgical studies (6).
Typically, these nodules represent incidental findings on chest X-rays or Chest CT performed for other reasons. Patients affected are generally asymptomatic, and when symptoms present they can be confused with concurrent medical conditions.
MPMNs have been shown to associate with many different pulmonary conditions, as well as with congestive heart failure (3). It has been suggested that they more often associate with chronic conditions than with acute lung injury. Accordingly, almost no reports have been provided on adolescents/children, making the hypothesis of a congenital origin less likely.
MPMNs did not show a clear lung, lobe or apical/basal distribution, nor age group differences, but showed a female prevalence, which is considered to be possibly related to their positivity for PR.
As aforementioned, they can present either as single pulmonary nodules or as multiple lung nodules, sometimes in a miliary pattern (6). In 2007, Suster and Moran (7) described five cases of MPMNs in a widespread bilateral distribution and drew parallels to meningioangiomatosis, a condition of the cerebral cortex and leptomeninges associated with neurofibromatosis type 2. Their observations prompted the current nomenclature “diffuse pulmonary meningotheliomatosis” to describe this rare condition. DPM, just as isolated MPMNs, has a predilection for females (92%), with a median age of 59.5, consistent with prior reports of prevalence in the sixth decade of life. The majority of patients with DPM are asymptomatic at the time of diagnosis (72%) (6).
Histopathological confirmation requires nodule sampling, most often obtained through surgical lung resection, most recently obtainable through video-assisted thoracic surgery (VATS) technique. Recently a possible role for bronchoscopic biopsies has been advocated, with possible reduction in adverse events (8).
The clinical relevance of MPMNs is still unclear. At present agreement is lacking on which should be considered the most appropriate management or follow up for these lesions. Thymic neoplasms are malignancies originating from thymic epithelium, the two main entities being thymoma and thymic carcinoma. Thymoma has an estimated incidence of about 1.5 cases per million people and the 5-year survival rate for all stages of thymoma of about 78% (9).
When disease is localized, guidelines recommend surgery with curative intent as treatment of choice, with a role for adjuvant radiotherapy and chemotherapy depending on the extent of resection, and a role for neoadjuvant chemotherapy in case of bulky lesions. For advanced, non resectable or metastatic disease chemoradiotherapy is recommended (10).
In our clinical case histological diagnosis of the mediastinal lesion raised the clinical suspicion of thymic neoplasm with pulmonary metastases. In the absence of pulmonary metastasis, the mediastinal lesion was considered suitable for radical surgical resection, for which a multidisciplinary discussion was advocated to discuss the most appropriated therapeutic approach for this case.
Conclusions
This clinical report underlines the importance of accurate definition of uncertain metastatic lesions in patients affected by thymoma with potentially resectable disease, not to discard the potential best treatment option for each single patient. In thymic neoplasms with pulmonary lesions of uncertain significance, meningothelial like nodules and diffuse pulmonary meningotheliomatosis should be considered among the differential diagnoses.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Korn D, Bensch K, Liebow AA, et al. Multiple minute pulmonary tumors resembling chemodectomas. Am J Pathol 1960;37:641-72. [PubMed]
- Zak FG, Chabes A. Pulmonary chemodectomatosis. JAMA 1963;183:887-9. [Crossref] [PubMed]
- Churg AM, Warnock ML. So-called "minute pulmonary chemodectoma": a tumor not related to paragangliomas. Cancer 1976;37:1759-69. [Crossref] [PubMed]
- Gaffey MJ, Mills SE, Askin FB. Minute pulmonary meningothelial-like nodules. A clinicopathologic study of so-called minute pulmonary chemodectoma. Am J Surg Pathol 1988;12:167-75. [Crossref] [PubMed]
- Travis WD, Colby TV, Corrin B, et al. Histological typing of lung and pleural tumours. Berlin: Springer, 1999.
- Gleason JB, Valentin R, Almeida P, et al. Diffuse pulmonary meningotheliomatosis: A literature review of a rare diffuse parenchymal lung disease with unclear clinical significance. J Assoc Chest Physicians 2017;5:18-25. [Crossref]
- Suster S, Moran CA. Diffuse pulmonary meningotheliomatosis. Am J Surg Pathol 2007;31:624-31. [Crossref] [PubMed]
- Bernabeu Mora R, Sánchez Nieto JM, Hu C, et al. Diffuse pulmonary meningotheliomatosis diagnosed by transbronchial lung biopsy. Respiration 2013;86:145-8. [Crossref] [PubMed]
- Rashid OM, Cassano AD, Takabe K. Thymic neoplasm: a rare disease with a complex clinical presentation. J Thorac Dis 2013;5:173-83. [PubMed]
- Gubens MA. Treatment updates in advanced thymoma and thymic carcinoma. Curr Treat Options Oncol 2012;13:527-34. [Crossref] [PubMed]


