Clinical predictors of the effectiveness of tiotropium in adults with symptomatic asthma: a real-life study
Introduction
The management of asthma is still challenging. Despite the current guidelines and effective treatment options for asthma, the control rate of asthma was around 50% and 70% in the real world and randomized controlled trials (RCTs), respectively (1,2). Asthma is a complex chronic inflammatory airway disease characterized by airway inflammation, reversible airway obstruction, airway hyper responsiveness. Low-dose inhaled corticosteroids (ICS) and ICS/long-acting B2-agonist (LABA) bronchodilators are the cornerstone therapy for asthma according to Global Initiative for Asthma (GINA) guidelines at steps 1 to 3. The once-daily long-acting muscarinic antagonist (LAMA) tiotropium is another bronchodilator. It is widely used for chronic obstructive pulmonary disease (COPD) and may be beneficial for asthma control including airway smooth muscle relaxation, anti-inflammatory properties (3), preventing airway from remodeling (4), inhibiting mucus gland hypertrophy, decreasing the number of goblet cells, and reducing airway smooth muscle thickening (5,6). Therefore, tiotropium is included as an add-on therapy for people with poorly-controlled asthma at steps 4 and 5 in GINA guideline 2016.
Several studies showed that tiotropium improved symptom control and lung function in mild-to-moderate asthmatic patients treated with ICS alone (7-11), and tiotropium was proven non-inferior to salmeterol as an add-on to ICS (7-9). The results were especially in patients with high cholinergic tone, which presented with lower resting heart rate but increased airway obstruction (12). Two large phase III, randomized, double-blind, placebo-controlled, parallel-group trials indicated that tiotropium add-on to the therapy for severe asthma patients with medium-to-high-dose ICS plus LABA significantly improved lung function, increased the time to asthma exacerbation, and the first episode of asthma worsening (13). Another phase III trial indicated that tiotropium significantly improved lung function and symptoms in Japanese patients with symptomatic asthma despite under ICS with or without LABA (14).
The efficacy and safety of tiotropium across asthma severity have been investigated in a number of studies, but asthma is a heterogeneous disease which may respond differently to targeted therapies due to different asthma phenotypes (15). Asthma management should be individualized according to the phenotypic characteristics of the patients. It was not clear that who can get benefit from tiotropium add-on therapy. Limited studies were available to determine whether there were clinical characteristics that could predict the responses to tiotropium add-on therapy. A subgroup analysis from the two phase III PrimoTinA-asthma studies (13) showed that tiotropium provided an effective add-on therapy, regardless of age, sex, body mass index (BMI), forced expiratory volume in one second (FEV1%), serum IgE levels and eosinophils counts (16). Peters et al. reported that the predictors of positive clinical response to tiotropium included positive albuterol response and airway obstruction (12). However, in addition to these two randomize controlled trials (RCTs), studies investigating tiotropium as add-on therapy in real-life were also needed. In real-world practice, asthmatics with several comorbidities, such as current smoker were present. In RCT, several factors not present in the controlled setting can affect the eventual outcomes. The purpose of this study was to point out whether any clinical characteristics may be used to predict if asthmatic patients could benefit from tiotropium add-on therapy in real-life clinical practice.
Methods
Study design
The study was performed retrospectively at the Division of Pulmonary and Critical Care Medicine, China Medical University Hospital, a 2,146-bed community-based university hospital in Taichung, Taiwan between July 2016 and July 2017. The study was approved by the China Medical University Hospital Institutional Review Board (CMUH103-REC1-112), and written consent was obtained from all patients.
Study cohort and data collection
Patients were included into this study if they met the following criteria: (I) aged >18 years; (II) the diagnosis of asthma was not only based on the clinical symptoms, but proved by a reversibility of airflow limitation on spirometry (increase in FEV1 >12% and 200 mL after 400 µg of salbutamol administration); (III) poorly controlled by maintenance treatment using low- or medium-to-high-dose of ICS with LABA for 4 weeks or more, which indicated the need of step-up treatment; (IV) tiotropium bromide was taken as add-on therapy with a recommended dose of 5 µg once daily via the Respimat Soft MoistTM inhaler. According to GINA 2016, add-on therapy with tiotropium is other controller option in patients treated as step 3 in whom adequate asthma control has not been achieved with low dose ICS/LABA combination. And it is preferred controller option in those treated as step 4 in whom well asthma control has not been achieved with medium to high dose ICS/LABA combination. In Taiwan, although there is no strong evidence for the side effects of medium to high dose of ICS, a lot of patients were afraid of it. Therefore, we provide another controller option (tiotropium add-on therapy at step 3 to step 4 and step 4 to step 5) to improve asthma control and try to find out predictors of the effectiveness of tiotropium add-on therapy in symptomatic asthmatics.
Every asthmatic all participated in pay-for-performance (P4P) scheme as a management program result into improved patient health outcomes and into healthcare cost savings. Self-management for asthma in P4P scheme include patient education, active communication of patients with physicians and nurses, monitoring the patient asthma symptoms regularly (ACT), and even maintaining diet and exercise programs. All of the potential predictors were collected, including age, sex, smoking history (yes or no), asthma and COPD overlap (ACO), BMI, gastroesophageal reflux disease (GERD), the steps of asthma control, pulmonary function test (PFT), asthma control test (ACT) score, eosinophil counts, and serum IgE level. ACO was defined based on Global Initiative for Asthma (GINA) 2017. It is characterized by persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD.
Assessments
Patients received asthma therapeutic options following the recommendations of the GINA guidelines. The ACT scores and PFT were used to assess the effectiveness of asthma control after add-on therapy. The ACT questionnaire was developed to assess asthma control since 2004 (17). Patients returned to our clinics after 3 months of add-on therapy. We divided the subjects into two groups depending on the improvement in ACT score: one group was tiotropium good responder (TGR), and the other was tiotropium poor responder (TPR). A difference of 3 points between mean ACT scores in 2 or more patient groups is a minimally important difference (MID) with clinical meanings. In contrast, a difference of mean scores between 2 samples less than 2 would not be clinically significant (18). According to this, TGR was defined as patients whose ACT score increased above 3 points after 3 months add-on therapy, while TPR referred to patients whose ACT score increased under 2 points or even decreased after 3 months add-on therapy.
After 3 months tiotropium add-on therapy, patients in TGR group remained in the treatment with tiotropium. In TPR group, the patients whose asthma was not controlled using low-dose ICS plus LABA (GINA step 3) were switched to medium-to-high-dose ICS plus LABA, and the others whose asthma was not controlled using medium-to-high-dose ICS plus LABA (GINA step 4) were switched to systemic corticosteroid or other alternative therapy such as theophylline, leukotriene receptor antagonists (LTRAs), and anti-immunoglobulin E (IgE) antibody. We hypothesized that there were some differences in patient clinical characteristics between TGR and TPR groups.
Statistical analysis
The data were analyzed using SPSS for Windows, version 17.0. Continuous variables were reported as mean ± SD and were compared using 2-tailed student’s t-tests. Categorical variables were reported as the numbers of patients and percentages. Differences between categorical variables were evaluated using Chi-square or Fisher’s exact test. Univariate analysis was performed to determine factors that were associated with asthma patient’s positive response to tiotropium. Variables were entered into a final model using a stepwise multiple logistic regression analysis. All statistical tests were 2-sided; a P value <0.05 was considered significant.
Results
Patient characteristics
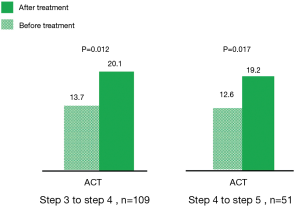
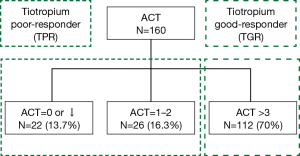
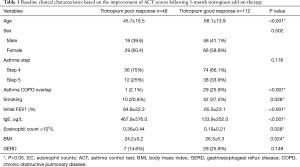
Between July 2016 and July 2017, 160 patients with symptomatic asthma lasting for 4 weeks or longer, despite being prescribed regular maintenance therapy, needed step-up treatment. Among them, 109 patients had been receiving low-dose ICS plus LABA (GINA step 3), and 51 patients had been receiving medium-to-high-dose ICS plus LABA (GINA step 4). After 3 months of tiotropium add-on therapy, the ACT score increased from 13.7 to 20.1 points in the former group (GINA step 3) (P=0.012) and from 12.6 to 19.2 points in the latter group (GINA step 4) (0.017) (Figure 1). These patients were divided into two groups based on the improvement of ACT score. One hundred and twelve patients (70%) had good response to tiotropium (TGR group), whose ACT score increased above 3 points. 48 patients (30%) had poor response to tiotropium (TPR group), whose ACT score increased less than 3 points. Among them, 26 patients (16.3%) increased ACT score by 1 to 2 points and 22 patients (13.7%) whose ACT score decreased or did not change (Figure 2). There were 138 patients (86.3%) with increase of ACT score. Baseline characteristics of these patients were shown in Table 1. Significant differences in age, ACO patients, cigarette use, initial FEV1, serum IgE level, eosinophil count, and BMI were found between these two groups. TGR group not only was older than TPR group (mean, 68.1±13.9 vs. 45.7±16.5 years; P<0.001), but had a higher proportion of cigarette use (42/112, 37.5%) than TPR group (10/48, 20.8%) (P=0.028). Therefore, there were more ACO patients (29/112, 25.9%) in TGR group than in TPR group (1/48, 2.1%) (P<0.001). The initial FEV1 of TGR group was significantly lower than TPR group (65.5%±23.1% vs. 84.8%±22.3%; P<0.001). Similarly, serum total IgE level and absolute eosinophil count in TGR group were also lower than those of TPR group [serum IgE level: 133.9±252.0 vs. 467.9±576.0 µg/L; P<0.001; eosinophil count: (0.19±0.21)×109/L vs. (0.36±0.44)×109/L; P=0.028]. However, the BMI in TGR group was higher than in TPR group (26.3±5.3 vs. 24.2±5.2; P=0.024).
Full table
Predictors of response to tiotropium
Univariate and multivariate logistic regression analyses were performed to determine whether clinical characteristics were predictors of good or poor response to tiotropium. These potential parameters included age, sex, asthma treatment step, ACO, cigarette use, initial FEV1, serum IgE level, eosinophil count, BMI and GERD. After univariate analysis, clinical predictors of good response to tiotropium were found in patients with old age (age >65 years) (OR =4.89), ACO (OR =16.42), cigarette use (OR =2.95), initial FEV1 <80% (OR =6.92), and BMI >30 (HR =4.54). Patients with high serum IgE level >430 µg/L (OR =0.16) and eosinophil counts >0.6×109 (OR =0.25) had a negative impact on responses to tiotropium. Although age, sex, cigarette use, ACO, initial FEV1 and serum eosinophil were predictors in univariate analysis and lost significance in multivariate analysis. These variables cannot be regarded as predictors of good or poor response to tiotropium. However, most patients (86.3%) with symptomatic asthma still can get benefit from tiotropium add-on therapy. Moreover, patients with poor response to tiotropium add-on therapy had serum IgE level >430 µg/L in multivariate analysis (Table 2).

Full table
Discussion
Our findings showed that tiotropium as add-on therapy to maintenance treatment with ICS plus LABA in patients with symptomatic asthma improved asthma control regardless of age, sex, cigarette use, ACO, initial FEV1, serum eosinophil count, and BMI. The ACT score increased from 13.7 to 20.1 points and from 12.6 to 19.2 points in GINA steps 3 and 4 patients, respectively. The independent predictor of poor response to tiotropium add-on therapy was IgE level >430 µg/L. The results were consistent with previous phase III RCTs (9,13,14,16). However, those RCTs included highly selective patients under strictly-controlled conditions and perhaps were not indicative of actual asthma patients in our real clinical practice. There were several real-life studies published regarding this issue. Prince et al., showed the efficacy of LAMAs as add-on therapy was associated with significant decrease in asthma exacerbation and antibiotics prescriptions in real-life asthma care (19). The other real-life study reported that tiotropium add-on therapy in severe asthmatics improved lung function significantly and reduced the number of emergency department visits and hospitalizations (20). To our knowledge, this was the first real-life study using ACT to evaluate the effectiveness of tiotropium as add-on therapy in moderate-to-severe asthmatics, and the results showed that the high serum total IgE level was associated with the negative predictor of this treatment.
Anticholinergics may be better for the following groups: elderly, patients with intolerance to B2 agonist, and chronic and fixed airway obstruction asthma (21,22). A recent study in South Korea indicated that tiotropium offered a good option for the treatment of elderly patients, especially for those with cardiovascular diseases since a large number of medicines were related to unfavorable side effects for the elderly (23). In our results, elderly patients seemed to benefit from tiotropium better than younger adults. However, age >65 years old was not a predictor of good response to tiotropium in our multivariate analysis (P=0.094). The overlap between asthma and COPD (ACO) was associated with a good response to anticholinergics, which could have proportionately greater effect than B2-agonists in COPD patients (24). Cigarette use contributes to the oxidative stress, which alters the histone acetylation (25) and changes the inflammatory mechanisms in asthma, and thereby becomes similar to that seen in COPD with increasing CD8 cells and neutrophils (26). This could be the reasons of poor response to corticosteroids in some smoker with asthma (27). Patients with severe asthma were positively correlated with neutrophil counts in sputum analysis. Tiotropium offers an excellent and sustained bronchodilator effect in non-eosinophilic phenotype asthmatics (28), which blocks the M1 and M3 anticholinergic receptors on the airway to reduce smooth muscle tone and result in bronchodilation (29). Our univariate analysis results were consistent with these findings, but did not reach significant outcomes in multivariate analysis. However, patients in TGR group not only were elder than those in TPR group, but had a higher proportion of cigarette use than TPR group. Therefore, there were more ACO patients in TGR group than in TPR group. According to that, if patients with asthma poorly controlled by current maintenance therapy were aged >65 years or had been exposed to cigarette smoke or were ACO, they may be potential good candidates for treatment with tiotropium as an add-on therapy.
Peters et al., indicated that decreased FEV1/FVC ratio and higher cholinergic tone were good predictors of positive response to tiotropium, but other baseline parameters were not (12). In our results, Initial FEV1 <80% was not an independent positive response predictor of tiotropium add-on therapy. Although Peters et al., used peak expiratory flow (PEF) as primary outcome, and our study used ACT, Park et al., reported that ACT does not always correlate with lung function parameters. Such results may be explained by the difference of outcome measures. We should assess asthma control by patients’ clinical characteristics and comorbidities along with ACT scores and lung function (30). Obese patients with asthma do not respond well to standard controller therapy (31). There were changes in neurological control of airway smooth muscle tone through cholinergic signals pathway among obese patients (32). Besides, high serum insulin levels in obese patients increased vagal-induced bronchoconstriction through acetylcholine release and vagal signaling (33). According to this background information, tiotropium should offer clinical advantages in obese patients or those individuals characterized by an increased cholinergic activity. Our results were similar to previous findings. BMI >30 was a potential positive predictor of tiotropium as add-on therapy in univariate analysis, but not in multivariate analysis. The reason may be that the number of patients with BMI >30 was too small to find significance in our study.
The subgroup analysis in the two phase III PrimoTinA-asthma studies (13) showed that tiotropium improved asthma control irrespective of age, sex, BMI, FEV1% predicted at screening, serum total IgE level and eosinophils counts (16). These results were consistent with our findings, except serum total IgE level. In subgroup analysis by asthma control questionnaire (ACQ) from this study, cigarette use status, FEV1(%), and blood serum eosinophil counts may influence the effect of tiotropium on clinical responses. We believed that subgroup categorization increased the variation of results within subgroups and the likelihood of spurious interactions and a post hoc subgroup analysis did not have sufficient power for such analyses.
Our study indicated that the serum total IgE level >430 µg/L in patients with uncontrolled asthma was an independent predictor of poor response to tiotropium as add-on therapy. These patients were considered to belong to high type 2 phenotype, which was related to increasing airway tone and serum eosinophilia, high IgE level and expression of cytokines (interleukin-4, -5, and -13) in tissues (34). Serum eosinophil counts also served as an initial biomarker to predict response to ICS and biologic agents such as anti-interleukin-4, -5, -13 and anti-IgE. The anti-IgE or anti-interleukin-5 monoclonal antibody was usually reserved for severe asthmatics with high serum total IgE level and eosinophil counts but poorly-controlled by high-dose ICS plus LABA. The current study showed that patients in TPR group had a higher serum total IgE level and eosinophil counts than TGR groups. Therefore, tiotropium add-on therapy may not be the first choice in patients with elevated serum total IgE, especially in serum total IgE >430 µg/L. Before adding on tiotropium to standard therapy, we must try to find out some reasons, such as persistent allergen exposure, drug adherence, inhaler technique, comorbidity, even severe allergic asthma or severe eosinophilic asthma why asthmatics with persistent elevated serum total IgE or eosinophil counts after control therapy. These patients in TPR group may be candidate for high dose corticosteroids or biologic agents rather than tiotropium after excluding above reasons.
There were some limitations in our study. First, the numbers of patients in our study were small, and the patient number in TGR and TPR groups were imbalanced due to the lack of control group. Since we retrospectively observed the effectiveness of tiotropium as add on therapy in real-life patients with symptomatic asthma, there was no opportunity to randomize patients into two equal groups. The patients served as their own controls, and the clinical characteristics were found different between TGR and TPR groups. Second, we used mainly ACT to evaluate the effectiveness of tiotropium. This was different from previous studies which used lung function or asthma acute exacerbation as outcome measurement. We believed that the ACT was a convenient and useful tool to evaluate asthma control by caregivers and patients. However, the event of asthma exacerbation was not easy to record and detect in a retrospective observational study. We also did not record the quality of life (QoL) and PFT parameters. According to the health insurance system in Taiwan, PFT were not allowed to perform every 3 months. The assessment of asthma control should be based on not only patients’ baseline characteristics, ACT score, but lung function. Third, cigarette use was not clearly defined. Although cigarette use significantly affected bronchial inflammation and epithelial change, the epithelial changes in airway of an ex-smoker was similar to those non-smokers. We should clearly define the smoking status such as current smoker or ex-smoker. Fourth, our study did not describe the atopic status, such as allergic rhinitis (AR) and allergic dermatitis (AD) of each patient. Many patients with allergic disease have elevated level of total IgE. The usefulness of measuring the total IgE level in AR or AD is very limited. However, allergic asthma severity correlates with increased total IgE level, regardless of atopy status. Fifth, it was not easy to normalize eosinophil counts for every asthmatic before tiotropium add on-therapy in retrospective study. At least, the eosinophil counts in symptomatic asthmatics were collected after asthma treatment and the need to step-up treatment. Sixth, we did not provide the adherence rate in the study. However, the poor adherence rate between these two groups (TGR and TPR) may not be significantly difference because all asthmatics participated in P4P scheme. Finally, as this was not a prospective and randomized control study, there might be some confounding factors, biases or unknown factors affecting the results during the analysis.
Conclusions
In real-life clinical care practice, tiotropium may be the effective add-on therapy in patients with uncontrolled asthma despite the use of low- or medium-to-high-dose of ICS plus LABA and regardless of age, sex, cigarette use, ACO, initial FEV1, serum eosinophil count, and BMI. The independent predictor of poor response to tiotropium add-on therapy was serum total IgE level >430 µg/L. In conclusion, tiotropium add-on therapy may not be the first choice in symptomatic asthmatics with serum total IgE >430 µg/L. However, future large prospective studies are warranted to confirm the results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the China Medical University Hospital Institutional Review Board (CMUH103-REC1-112), and written consent was obtained from all patients.
References
- Price D, Fletcher M, van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med 2014;24:14009. [Crossref] [PubMed]
- Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med 2004;170:836-44. [Crossref] [PubMed]
- Beeh KM, Moroni-Zentgraf P, Ablinger O, et al. Tiotropium Respimat(R) in asthma: a double-blind, randomised, dose-ranging study in adult patients with moderate asthma. Respir Res 2014;15:61. [Crossref] [PubMed]
- Kistemaker LE, Gosens R. Acetylcholine beyond bronchoconstriction: roles in inflammation and remodeling. Trends Pharmacol Sci 2015;36:164-71. [Crossref] [PubMed]
- Bos IS, Gosens R, Zuidhof AB, et al. Inhibition of allergen-induced airway remodelling by tiotropium and budesonide: a comparison. Eur Respir J 2007;30:653-61. [Crossref] [PubMed]
- Gosens R, Bos IS, Zaagsma J, et al. Protective effects of tiotropium bromide in the progression of airway smooth muscle remodeling. Am J Respir Crit Care Med 2005;171:1096-102. [Crossref] [PubMed]
- Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med 2010;363:1715-26. [Crossref] [PubMed]
- Bateman ED, Kornmann O, Schmidt P, et al. Tiotropium is noninferior to salmeterol in maintaining improved lung function in B16-Arg/Arg patients with asthma. J Allergy Clin Immunol 2011;128:315-22. [Crossref] [PubMed]
- Kerstjens HA, Casale TB, Bleecker ER, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respir Med 2015;3:367-76. [Crossref] [PubMed]
- Beeh KM, Moroni-Zentgraf P, Ablinger O, et al. Tiotropium Respimat® in asthma: a double-blind, randomised, dose-ranging study in adult patients with moderate asthma. Respir Res 2014;15:61. [Crossref] [PubMed]
- Paggiaro P, Halpin DM, Buhl R, et al. The Effect of Tiotropium in Symptomatic Asthma Despite Low- to Medium-Dose Inhaled Corticosteroids: A Randomized Controlled Trial. J Allergy Clin Immunol Pract 2016;4:104-13.e2. [Crossref] [PubMed]
- Peters SP, Bleecker ER, Kunselman SJ, et al. Predictors of response to tiotropium versus salmeterol in asthmatic adults. J Allergy Clin Immunol 2013;132:1068-74.e1. [Crossref] [PubMed]
- Kerstjens HA, Engel M, Dahl R, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med 2012;367:1198-207. [Crossref] [PubMed]
- Ohta K, Ichinose M, Tohda Y, et al. Long-Term Once-Daily Tiotropium Respimat(R) Is Well Tolerated and Maintains Efficacy over 52 Weeks in Patients with Symptomatic Asthma in Japan: A Randomised, Placebo-Controlled Study. PloS One 2015;10. [Crossref] [PubMed]
- Agache I, Akdis CA. Endotypes of allergic diseases and asthma: An important step in building blocks for the future of precision medicine. Allergol Int 2016;65:243-52. [Crossref] [PubMed]
- Kerstjens HA, Moroni-Zentgraf P, Tashkin DP, et al. Tiotropium improves lung function, exacerbation rate, and asthma control, independent of baseline characteristics including age, degree of airway obstruction, and allergic status. Respir Med 2016;117:198-206. [Crossref] [PubMed]
- Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol 2004;113:59-65. [Crossref] [PubMed]
- Schatz M, Kosinski M, Yarlas AS, et al. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol 2009;124:719-23.e1. [Crossref] [PubMed]
- Price D, Kaplan A, Jones R, et al. Long-acting muscarinic antagonist use in adults with asthma: real-life prescribing and outcomes of add-on therapy with tiotropium bromide. J Asthma Allergy 2015;8:1-13. [PubMed]
- Abadoglu O, Berk S. Tiotropium may improve asthma symptoms and lung function in asthmatic patients with irreversible airway obstruction: the real-life data. Clin Respir J 2016;10:421-7. [Crossref] [PubMed]
- Cazzola M, Centanni S, Donner CF. Anticholinergic agents. Pulm Pharmacol Ther 1998;11:381-92. [Crossref]
- Boulet LP, Becker A, Bérubé D, et al. CMAJ 1999;161:SF1-14. [Summary of the recommendations of the Canadian Consensus Conference on Asthma 1999. Canadian Asthma Consensus Group]. [PubMed]
- Ban GY, Ye YM, Lee Y, et al. Predictors of Asthma Control by Stepwise Treatment in Elderly Asthmatic Patients. J Korean Med Sci 2015;30:1042-7. [Crossref] [PubMed]
- Quirce S, Dominguez-Ortega J, Barranco P. Anticholinergics for treatment of asthma. J Investig Allergol Clin Immunol 2015;25:84-93. [PubMed]
- Chung KF, Marwick JA. Molecular mechanisms of oxidative stress in airways and lungs with reference to asthma and chronic obstructive pulmonary disease. Ann N Y Acad Sci 2010;1203:85-91. [Crossref] [PubMed]
- Haldar P, Pavord ID. Noneosinophilic asthma: a distinct clinical and pathologic phenotype. J Allergy Clin Immunol 2007;119:1043-52. [Crossref] [PubMed]
- Chaudhuri R, Livingston E, McMahon AD, et al. Cigarette smoking impairs the therapeutic response to oral corticosteroids in chronic asthma. Am J Respir Crit Care Med 2003;168:1308-11. [Crossref] [PubMed]
- Iwamoto H, Yokoyama A, Shiota N, et al. Tiotropium bromide is effective for severe asthma with noneosinophilic phenotype. Eur Respir J 2008;31:1379-80. [Crossref] [PubMed]
- Belmonte KE. Cholinergic pathways in the lungs and anticholinergic therapy for chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005;2:297-304; discussion 11-2. [Crossref] [PubMed]
- Park SY, Yoon SY, Shin B, et al. Clinical factors affecting discrepant correlation between asthma control test score and pulmonary function. Allergy Asthma Immunol Res 2015;7:83-7. [Crossref] [PubMed]
- Mosen DM, Schatz M, Magid DJ, et al. The relationship between obesity and asthma severity and control in adults. J Allergy Clin Immunol 2008;122:507-11.e6. [Crossref] [PubMed]
- Nie Z, Jacoby DB, Fryer AD. Hyperinsulinemia potentiates airway responsiveness to parasympathetic nerve stimulation in obese rats. Am J Respir Cell Mol Biol 2014;51:251-61. [PubMed]
- Leiria LO, Arantes-Costa FM, Calixto MC, et al. Increased airway reactivity and hyperinsulinemia in obese mice are linked by ERK signaling in brain stem cholinergic neurons. Cell Rep 2015;11:934-43. [Crossref] [PubMed]
- Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 2009;180:388-95. [Crossref] [PubMed]


