Multimodality therapy in subclassified stage IIIA–N2 non-small cell lung cancer patients according to the Robinson classification: heterogeneity and management
Introduction
Lung cancer is the most common cause of cancer-related death worldwide. In men, the highest incidence rates are observed in Eastern Europe and North America, and in women, high incidence rates are observed in North America and northern and western Europe (1). Non-small cell lung cancer (NSCLC) with mediastinal lymph node involvement (N2) is a heterogeneous entity in stage III. Approximately 30% of patients newly diagnosed with NSCLC are classified as N2-positive stage IIIA. The Robinson classification subdivides these N2-patients into four groups depending on the extent of mediastinal lymph node invasion (IIIA1–IIIA4) (2). Many different strategies have been evaluated to treat patients with stage IIIA NSCLC, reflecting N2 disease, but no clear consensus on the management of these patients exists (3). Treatment guidelines suggest that indication and therapy should be performed in tumour centres. Treatment approaches require an interdisciplinary discussion, decisions for reliable indication prior to treatment and adequate staging prior to surgery (conference with documentation and the participation of at least pneumology, oncology, thoracic surgery, radiation oncology, and diagnostic radiology). Particularly in advanced stage IIIA3/IIIA4 cancers, these steps should be conducted at centres with appropriate experience (4).
The objective of this analysis was to investigate the results of strict multimodality treatment strategies for N2 patients determined by an interdisciplinary tumour board.
Methods
Study design
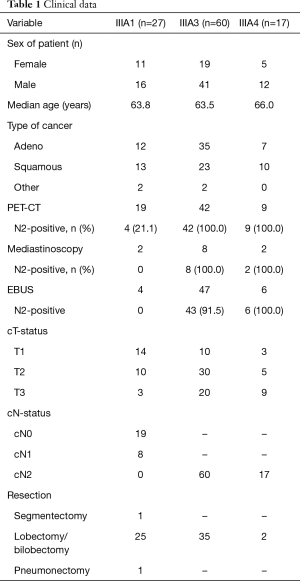
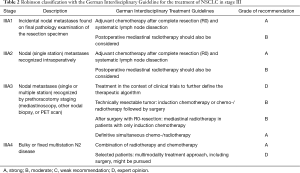
This study was a retrospective study and survival analysis of 104 consecutive patients with stage IIIA–N2 NSCLC subclassified according to the Robinson classification and treated within a multimodality treatment regime between January 2009 and June 2014. All patients were evaluated and discussed by an interdisciplinary lung tumour board. Compliance with the Interdisciplinary Guideline of the German Respiratory Society and German Cancer Society for the treatment of NSCLC, in effect at the time of diagnosis, was documented (Table 1) (4). Patients, whose performance status or other factors (side effects, tumour progress, second cancer) prevented participation in the indicated therapy according to the guidelines or patients who refused therapy were counted as deviations from the tumour board recommendation.
Full table
Mediastinal staging and Robinson classification
Most patients were initially staged using thoracic computed tomography (CT) scans, positron emission tomography (PET)-CT, or bone scans. Suspicious lymph nodes >1 cm on CT scan or a positive uptake value (>2.5) on PET scan identified during the preoperative staging workup were routinely biopsied. Pretreatment tissue confirmation of N2-disease was obtained through endobronchial/transoesophageal ultrasound transbronchial aspiration (EBUS/EUS-TBNA) or video-mediastinoscopy. Histological complete mediastinal staging was confirmed in stages IIIA3 and IIIA4 upon final histological examination of the resected specimen, which was postoperative for patients with N2-disease (IIIA1). Because we did not perform intraoperative mediastinal lymph node staging using a frozen sectioning technique, we did not classify IIIA2 tumours. Therefore, these patients were included in stage IIIA1.
After induction chemotherapy or chemo-/radiotherapy, patients were re-staged using CT-scans. The indications to proceed with surgical resection included tumour remission or stable tumour disease. A complete mediastinal lymphadenectomy was performed after anatomic resection of the tumour. Only the surgical margins were subjected to intraoperative frozen sectioning to determine the R0 status. Complete resection required all of the following: free resection margins proved microscopically; systematic nodal dissection, no extracapsular nodal extension of the tumor; and the highest mediastinal node removed was negative (5).
Collection of data and analysis of survival
Patient demographics, treatment strategy determined by the tumour board, course of treatment, operative mortality, survival and deviation from the tumour board recommendation were examined. Follow-up analysis was performed with recurrence and survival data documented in the tumour registry of the Tumour Centre Regensburg. Time to recurrence or progression-free interval and survival were calculated from the date of resection until recurrence, death or end of January 2017.
The primary endpoint of the study was to evaluate the impact of surgical resection within a multimodal treatment for N2-patients depending on the Robinson classification. The secondary endpoints included analysis of overall survival (OS) and survival with respect to the interdisciplinary recommendation of therapy.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA) for Windows (Microsoft Corp, Redmond, WA, USA). Descriptive statistics were used to describe patient characteristics and compare variables. Categorical data are shown as frequency distributions (n) and percentages (%). Kaplan-Meier analysis was used to plot survival curves, and the log-rank test was used to differences between subgroups. We estimated the hazard ratio for each subgroup using the Cox regression analysis. A P value of <0.05 was assumed to be statistically significant.
Results
Treatment strategies (recommendation, execution, deviation)
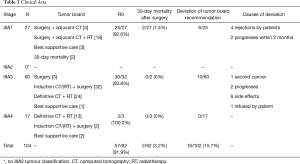
Between January 2009 and the end of June 2014, a total of 104 patients with stage IIIA (N2) NSCLC were treated in our centre for lung cancer. With strict interpretation of the German treatment guidelines, a match between these guidelines and the tumour conference recommendation was found in 94% of cases. In 6/104 patients (IIIA1 n=2, IIIA3 n=2, IIIA4 n=2), the recommendation of the tumour board was not correlated with the guidelines because of the limited patient performance status. Mortality within 30 days for all operated patients (n=61) was 3.2%.
The Robinson subgroups were IIIA1 (n=27; 63.8 years), IIIA3 (n=60; 63.5 years) and IIIA4 (n=17; 66.0 years) (Table 2).
Full table
All stage IIIA1 NSCLC cases were classified after surgical resection and pathological workup of all tissue specimens. Complete tumour resection (R0) was achieved in (25/27, 92.6%) of cases (n=25) in stage IIIA1 (Table 3). Final histological examination of the mediastinal lymph nodes (pN2) showed single-level invasion (involvement of only one mediastinal lymph node station) in 24 (88.9%) patients and multi-level pN2-disease (involvement of more than one mediastinal lymph node station) in three patients (11.1%) (Table 4). Only a single mediastinal lymph node was infiltrated by the tumour in 22 patients, while more than one lymph node were infiltrated in five patients (Table 4). Two IIIA1 patients died postoperatively (30-day mortality 7.4%). The tumour board recommended adjuvant chemotherapy (n=3) or chemo-/radiotherapy (n=19) for the remaining 25 patients. Three patients did not receive adjuvant treatment because of their reduced postoperative general conditions. The recommended therapy was followed in 19/25 (76.0%) cases (Table 3). Four patients rejected adjuvant chemotherapy or chemo-/radiotherapy. Reflecting tumour progression within 3 months, the suggested adjuvant therapy was not completed in two patients.
Full table

Full table
Stage IIIA3 was determined in 60 cases (mean age, 63.5 years). Two patients were suggested for curative resection without induction therapy. For both patients, the tumour board recommended postoperative radio-/chemotherapy. One patient refused adjuvant treatment, and the other patient only received incomplete chemotherapy because of pneumonia. One patient underwent surgery without induction therapy with a palliative intention. In these three patients without induction-therapy, two single-level patients and one multi-level pN2 patient were registered (Table 4).
In 32 patients, induction chemotherapy (n=16) or chemo-/radiotherapy (n=16) was performed. Anatomical lung resection with systematic lymphadenectomy was performed in 29/32 patients after induction therapy. A complete pathological response (ypT0N0) was seen in 10/29 (34.5%) patients. Histological downstaging of the mediastinal lymph nodes to regional lymph node involvement (ypN1) was observed in 3 (10.3%) patients; persistent single ypN2 was observed in 12 (41.4%) patients and multiple ypN2 in 4 (13.8%) patients (Table 4).
In three patients, the planned operation was cancelled because of tumour progression (n=1), side effects of induction treatment (n=1) and a synchronous second cancer (lymphoma; n=1). In 13/16 patients who only receive induction chemotherapy, adjuvant radiotherapy was postoperatively performed. One patient with postoperative tumour stage ypT0ypN0cM0 received no recommendation for adjuvant radiotherapy. In two patients, adjuvant radiotherapy was stopped because of side effects. Definitive simultaneous chemo-/radiotherapy was recommended in 24 patients, with full realization in 21 patients. In three patients, the planned definitive chemo-/radiotherapy schedule had to be stopped because of side effects (n=2) and tumour progression (n=1). One patient received the best supportive care.
All stage IIIA4 patients (n=17; mean age, 66.0 years) had, at baseline, an unresectable N2-disease with bulky mediastinal lymph node invasion. The tumour board recommended definitive radio-/chemotherapy for n=13 patients (76.5%) and the best supportive care for two patients. Two patients with initially recommended definitive chemo-/radiotherapy underwent surgery after a second tumour board decision because the tumour showed good response to this induction therapy. Both patients had persistent N2-disease (single-level yN2 n=1; multi-level yN2 n=1). One patient with multi-level pN2-disease underwent surgery during definitive chemo-/radiotherapy because of massive bleeding. In stage IIIA4 patients, no treatment deviations in correlation with the tumour board recommendations were documented.
Recurrence, recurrence-free survival and OS
Survival and risk factor analyses were conducted based on data from 100 patients. Patients with 30-day mortality (n=2) and cancer-unrelated deaths (n=2) were excluded from this calculation. The overall median survival for all patients (n=100) was 31.7 months. The 3- and 5-year survival rates were 43.5% and 30.5%, respectively.
The rate of complete resection (R0) was 96.8% (60/62 patients). Approximately 55.4% (n=31) of the remaining 56 patients had no tumour recurrence during follow-up. Local recurrences developed in 13 (23.2%) patients; 11 (19.6%) patients had distant metastases, and 1 (1.8%) patient had both distant metastasis and local recurrence. Patients with stage IIIA3 had twice as many distant metastases as patients with stage IIIA1 during follow-up (26.7% vs. 13%), but the local recurrence rates were nearly the same between both groups (23.3% vs. 26.1%). The median progression-free survival (PFS) of all R0 resected stage IIIA patients was 41.6 months. There was no significant difference (P=0.728) in PFS between subgroups IIIA1, IIIA3 and IIIA4.
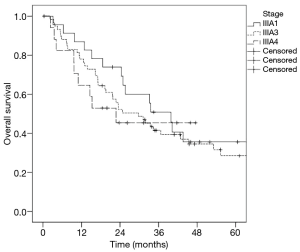
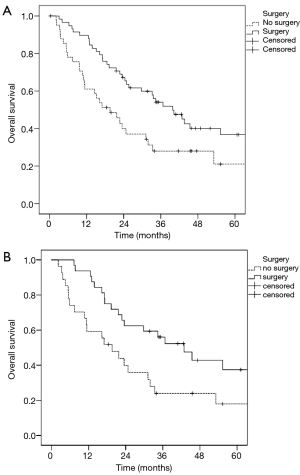
The median OS in stage IIIA1 patients was 39.8 months, in stage IIIA3 patients was 29.7 months and in stage IIIA4 patients was 22.7 months. There was no significant difference (P=0.583) in survival regarding the three Robinson subgroups (Figure 1). Patients who underwent surgical tumour resection had a significantly better median survival (39.8 vs. 19.6 months; P=0.014) compared to patients treated conservatively (Figure 2A). In addition, stage IIIA3 patients considered for surgery after induction therapy had significantly better median survival compared to non-surgically treated patients (42.8 vs. 19.6 months; P=0.026) (Figure 2B).
The comparison of infiltrated mediastinal lymph node stations showed a better prognosis for surgical patients with single-level invasion (median survival: 40.3 months) than in surgical patients with multi-level invasion (median survival: 24.9 months), but this difference was not significant (P=0.23). The number of infiltrated mediastinal lymph nodes (single vs. multiple) showed no significant influence (P=0.108) on OS, although a better prognosis, was demonstrated for patients with only one infiltrated mediastinal lymph node (median survival: 40.7 vs. 24.9 months).
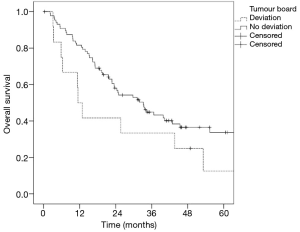
In n=88 (88%) cases, the treatment suggested by the tumour board was consistent with the treatment performed. Deviation from the interdisciplinary recommended therapy (n=12) led to a reduced median survival (11.4 vs. 31.8 months; P=0.137) compared to implementation of the suggested treatment approach (n=88) (Figure 3).
Discussion
Treatment of patients with mediastinal lymph nodes has been extensively discussed in thoracic surgery, and there is no clear consensus on the management of patients with stage IIIA lung cancer worldwide. The presence and extent of mediastinal lymph node invasion are strong prognostic factors in patients with NSCLC. The reason why N2-positive NSCLC exhibits a heterogeneous prognosis is that this group contains both local and systemic disease (6). The present study showed that all patients with N2-positive stage IIIA NSCLC who underwent multimodality therapy, including surgical resection, had significantly prolonged OS, even in advanced mediastinal involvement according to Robinson stage IIIA3 and IIIA4. Therefore, all patients in stage IIIA3 and IIIA4 should be evaluated for surgery depending on their response to induction therapy.
The prevalence of occult N2 metastases is, despite modern diagnostics with PET-CT, nearly 7.0% to 13%. Central tumours, solid T2 tumours, vascular invasion and adenocarcinoma are risk factors for a higher incidence for occult mediastinal lymph node metastases (7-9). The present analysis of patients with occult mediastinal metastases (stage IIIA1) showed the best median survival (39.8 months) for all N2 subgroups and only a recurrence rate of nearly 40%. The recurrences were predominantly loco-regional, suggesting that IIIA1 is a local disease. Distant metastases only developed in 13% of patients during follow-up. Honguero Martínez et al. showed comparable disease-free survival rates and a mean OS for occult N2 disease (IIIA1) of 36.0 and 38.9 months, respectively (10). In another study, the median OS rates with unsuspected N2-disease reached up to 76 months (11). Although these differences were statistically not relevant, the number of infiltrated mediastinal lymph node stations and number of infiltrated lymph nodes influenced the survival prognosis. In the present study nearly 90% of patients had single-level mediastinal lymph node invasion, and in nearly 80% of patients, only one N2-lymph node was infiltrated. Thus, mediastinal lymph node infiltration is often limited in stage IIIA1 patients. Unsuspected single-station N2 disease has a better survival than unsuspected multiple-station N2 disease (11). We observed a median OS for all operated patients with single-station N2-disease of 40.3 and 24.9 months for multiple-station N2-disease. Cho et al. reported that patients with stage IIIA1 single-station N2-disease had the best 5-year survival rate of 66.6% (36.4% for unsuspected multiple-station N2-disease) (11). Risk factors for unsuspected multiple-station N2-disease were histological proven adenocarcinoma, clinical N1-disease, a tumour size exceeding 3 cm and location of the primary tumour in the right middle lobe (11). In addition to multi-station N2-disease, the number of metastatic N2 lymph nodes (e.g., more than three) and non-regional N2 metastasis were independent risk factors for reduced recurrence-free survival (12). In the present study, patients with multiple infiltrated mediastinal lymph nodes had a worse OS compared to patients with single N2 lymph node infiltration.
Adjuvant chemotherapy confers an OS benefit for occult N2 nodal involvement, which was discovered postoperatively (13,14). Yang et al. saw no survival benefit for an additional adjuvant radiotherapy in stage IIIA1 (15). But the topic of postoperative irradiation in stage IIIA1 is still under debate (16).
Thus, we propose that the need for routine intraoperative pathological evaluation of mediastinal nodes for the determination of occult N2-disease (stage IIIA2) does not exist, as IIIA2 status affects neither the operative nor postoperative therapy compared to IIIA1. The positive results obtained for all surgically treated N2 patients suggest that continuing with anatomical resection is appropriate, particularly when a surgeon encounters occult pN2-disease during surgery. Proceeding with resection is clearly justified when complete resection is possible, particularly in the case of T1–2 tumour, single-level lymph node involvement and patients with cN0–1 tumours (17).
Otherwise, the role of surgical resection in patients with minimal clinical stage IIIA–N2 positive lung cancer (IIIA3) is controversial, particularly because of the variability in short- and long-term outcomes. Previous reports have demonstrated that neoadjuvant chemotherapy and/or radiotherapy followed by surgical resection provides better PFS than definitive chemoradiotherapy for patients with stage IIIA–N2 NSCLC (18,19). Induction chemotherapy offers downstaging and improved survival. A previous meta-analysis showed a 5% absolute benefit of preoperative chemotherapy on 5-year survival in patients with resectable NSCLC (20). This finding is consistent with the results of the present study, in which median OS was 29.7 months for all stage IIIA3 patients and 39.8 months for the subgroup of surgically treated patients. Thus, in the present study, patients in stage IIIA3 who underwent surgery had the same median survival as patients in stage IIIA1. Honguero Martínez et al. reported a similar prognosis for patients with single-station N2-disease treated with surgical resection and mediastinal lymphadenectomy as first-line treatment compared to those with occult N2-disease (10).
However, there is clear evidence of a benefit of preoperative chemotherapy regarding the time to distant recurrences of 10% at 5 years (from 60% to 70%) (20). The switch from a local to a systemic disease is based on the occurrence of clinical mediastinal lymph node involvement. Patients with stage IIIA3 tumours had twice as many distant metastases as patients with stage IIIA1 tumours during follow-up. Survival in patients with stage IIIA3 was likely determined by distant metastasis than local recurrence. However, the distribution of local and distant metastases between stage IIIA1 and IIIA3 is not a question at the time of chemotherapy application (preoperative vs. postoperative), as studies have shown that preoperative chemotherapy has a greater impact on distant metastases, whereas postoperative chemotherapy impacts local control (20). Despite neoadjuvant chemotherapy, patients with stage IIIA3 tumours had more frequent distant metastases than patients with occult N2-disease. Thus, clinical mediastinal lymph node infiltration might indicate the beginning of a systemic disease. This systemic effect is also reflected in the number of patients (nearly 50% of our stage IIIA3 patients) who are treated with chemo-/radiotherapy without surgery.
Patients in stage IIIA4 with bulky or fixed multi-station N2-disease had the lowest median OS (22.7 months) within the study sample. Li et al. showed nearly the same median survival time (23 months) for patients with stage IIIA (N2-bulky) or stage IIIB (T4N1–2M0), although in this study, the resection rate after chemo-/radiotherapy was much higher (62.5%) that that used in the present study (21). Lee et al. showed that neither an initial bulky nor extent of cN2 disease influenced prognosis (22). Patients with stage III NSCLC treated with surgery after induction chemo-/radiotherapy and with a pathologically complete lymph node response (ypN0) showed significantly higher survival. In the clinical trial of Meerbeeck et al., for patients with pathologically confirmed stage IIIA–N2 NSCLC and a response to induction chemotherapy, surgical resection did not improve overall (16.4 months) median survival compared with radiotherapy (17.5 months) (23). In the intergroup trial 0139, OS was better compared to that of Meerbeeck et al. and was also similar between groups that received induction chemo-/radiotherapy followed by surgery or consolidation with radiotherapy (23.6 vs. 22.2 months) (24). The difference in OS between the two studies (Intergroup 0139 27% vs. EORTC 15.7%) highlights the selection biases of each study. The Meerbeeck et al. study had multi-station N2-disease, whereas the intergroup trial was more likely to have single-station N2-disease. Also, for our stage IIIA3 patients, the majority had single-station involvement indicating that for most patients, mediastinal involvement was rather limited which implies a bias as prognosis is better for these specific patient categories.
The ESPATUE study showed that trimodality treatment that includes surgery and bimodality treatment without surgery but with a definitive chemoradiotherapy boost lead to excellent 5-year OS rates greater than 40% (25). These data show that chemotherapy plus radiotherapy is also an option for patients with clinical stage IIIA (IIIA3, IIIA4) NSCLC, particularly for patients with multiple/bulky disease, poor lung function or potential pneumonectomy.
Because treatment in stage IIIA lung cancer depends on many individual patient and tumour factors, the best management according to the guidelines and patient status can only be achieved by an interdisciplinary tumour board that includes a medical oncologist, radiation oncologist, and thoracic surgeon. Thus, we achieved a positive match between treatment recommendation and the German treatment guidelines in 94% of the discussed cases. The compliance with the tumour board recommendation showed better median OS rates compared to patients in which a deviation from the interdisciplinary recommended therapy was documented (31.8 vs. 11.4 months).
Conclusions
The primary task is careful preoperative staging to evaluate patients with clinical N2-disease, representing in most cases a systemic disease. In these cases, chemotherapy or chemo-/radiotherapy is indicated. Surgical resection should be recommended in specific cases of N2-disease (non-bulky, sensitivity to systemic treatment) because it contributes to satisfactory long-term survival. In patients treated with surgery and occult N2-invasion (stage IIIA1/IIIA2) or with relatively local N2-disease, adjuvant therapy should be administered when possible. This multimodal treatment provides the opportunity for a long recurrence-free interval and prolonged OS. All patients with N2-disease should be subclassified according to the Robinson classification, and the diagnostics and treatment options should be discussed by an interdisciplinary tumour board.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional ethics board of Hospital Barmherzige Brüder Regensburg.
References
- Tyczynski JE, Bray F, Parkin DM. Lung cancer in Europe in 2000: epidemiology, prevention, and early detection. Lancet Oncol 2003;4:45-55. [Crossref] [PubMed]
- Robinson LA, Ruckdeschel JC, Wagner H Jr, et al. Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:243S-65S.
- Veeramachaneni NK, Feins RH, Stephenson BJ, et al. Management of stage IIIA non-small cell lung cancer by thoracic surgeons in North America. Ann Thorac Surg 2012;94:922-6; discussion 926-8. [Crossref] [PubMed]
- Goeckenjan G, Sitter H, Thomas M, et al. Prevention, diagnosis, therapy, and follow-up of lung cancer. Interdisciplinary guideline of the German Respiratory Society and the German Cancer Society--abridged version. Pneumologie 2011;65:e51-75. [Crossref] [PubMed]
- Rami-Porta R, Wittekind C, Goldstraw P, et al. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25-33. [Crossref] [PubMed]
- Ichinose J, Murakawa T, Hino H, et al. Prognostic impact of the current Japanese nodal classification on outcomes in resected non-small cell lung cancer. Chest 2014;146:644-9. [Crossref] [PubMed]
- Bille A, Woo KM, Ahmad U, et al. Incidence of occult pN2 disease following resection and mediastinal lymph node dissection in clinical stage I lung cancer patients. Eur J Cardiothorac Surg 2017;51:674-9. [Crossref] [PubMed]
- Gao SJ, Kim AW, Puchalski JT, et al. Indications for invasive mediastinal staging in patients with early non-small cell lung cancer staged with PET-CT. Lung Cancer 2017;109:36-41. [Crossref] [PubMed]
- Yeh YC, Kadota K, Nitadori J, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification predicts occult lymph node metastasis in clinically mediastinal node-negative lung adenocarcinoma. Eur J Cardiothorac Surg 2016;49:e9-15. [Crossref] [PubMed]
- Honguero Martínez A, García Jiménez M, García Vicente A, et al. Is the prognosis of occult N2 disease similar to that of positive positron emission tomography-computed tomography (PET/CT) scan single-station N2 disease in patients with non-small cell lung cancer treated by surgical resection? Rev Esp Med Nucl Imagen Mol 2017;36:350-5. [Crossref] [PubMed]
- Cho HJ, Kim SR, Kim HR, et al. Modern outcome and risk analysis of surgically resected occult N2 non-small cell lung cancer. Ann Thorac Surg 2014;97:1920-5. [Crossref] [PubMed]
- Qiang G, Liang C, Yu Q, et al. Risk factors for recurrence after complete resection of pathological stage N2 non-small cell lung cancer. Thorac Cancer 2015;6:166-71. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. Survival of patients with unsuspected N2 (stage IIIA) nonsmall-cell lung cancer. Ann Thorac Surg 2008;86:362-6; discussion 366-7. [Crossref] [PubMed]
- Yanagawa J, Rusch VW. Current surgical therapy for stage IIIA (N2) non-small cell lung cancer. Semin Thorac Cardiovasc Surg 2011;23:291-6. [Crossref] [PubMed]
- Yang CF, Kumar A, Gulack BC, et al. Long-term outcomes after lobectomy for non-small cell lung cancer when unsuspected pN2 disease is found: A National Cancer Data Base analysis. J Thorac Cardiovasc Surg 2016;151:1380-8. [Crossref] [PubMed]
- Le Pechoux C, Dunant A, Faivre-Finn C, et al. Postoperative Radiotherapy for Pathologic N2 Non-Small-Cell Lung Cancer Treated With Adjuvant Chemotherapy: Need for Randomized Evidence. J Clin Oncol 2015;33:2930-1. [Crossref] [PubMed]
- Detterbeck F. What to do with "Surprise" N2?: intraoperative management of patients with non-small cell lung cancer. J Thorac Oncol 2008;3:289-302. [Crossref] [PubMed]
- Thomas M, Rube C, Hoffknecht P, et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol 2008;9:636-48. [Crossref] [PubMed]
- Gilligan D, Nicolson M, Smith I, et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet 2007;369:1929-37. [Crossref] [PubMed]
- NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014;383:1561-71. [Crossref] [PubMed]
- Li J, Dai CH, Yu LC, et al. Results of trimodality therapy in patients with stage IIIA (N2-bulky) and stage IIIB non-small-cell lung cancer. Clin Lung Cancer 2009;10:353-9. [Crossref] [PubMed]
- Lee H, Ahn YC, Pyo H, et al. Pretreatment clinical mediastinal nodal bulk and extent do not influence survival in N2-positive stage IIIA non-small cell lung cancer patients treated with trimodality therapy. Ann Surg Oncol 2014;21:2083-90. [Crossref] [PubMed]
- van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 2007;99:442-50. [Crossref] [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]
- Eberhardt WE, Pottgen C, Gauler TC, et al. Phase III Study of Surgery Versus Definitive Concurrent Chemoradiotherapy Boost in Patients With Resectable Stage IIIA(N2) and Selected IIIB Non-Small-Cell Lung Cancer After Induction Chemotherapy and Concurrent Chemoradiotherapy (ESPATUE). J Clin Oncol 2015;33:4194-201. [Crossref] [PubMed]