A prospective randomized trial of continuous paravertebral infusion versus intravenous patient-controlled analgesia after thoracoscopic lobectomy for lung cancer
Introduction
Since thoracoscopic lobectomy was introduced in 1990s, it has been regarded as a useful surgical option for early-stage lung cancer because it could reduce operative morbidities as compared with open lobectomy. However, some patients still complain of severe acute pain after thoracoscopic lobectomy, and the incidence of chronic pain was reported up to 30% (1,2). Penetration of the chest wall by trocars, torque at trocar, and compression of the intercostal nerves have all been suggested to cause pain after thoracoscopic lobectomy (3,4). Current analgesic options for acute pain include thoracic epidural analgesia, intravenous patient-controlled analgesia (iv-PCA), thoracic paravertebral block, and continuous paravertebral infusion (c-PVI) of local anesthetics. Among these methods, iv-PCA has been widely used because of its technical simplicity and relatively effective pain management (5). However, side effects such as sedation, nausea, and vomiting due to iv-PCA’s systemic delivery of analgesics preclude its continuous use. In contrast, c-PVI of local anesthetics thorough a catheter below the parietal pleura has been known to reduce side effects of iv-PCA and effectively control postoperative pain (6). Although c-PVI could provide effective pain relief in comparison with iv-PCA with better side-effects profile in patients who underwent open thoracotomy (7-11), only a few existing studies have compared their analgesic effects after thoracoscopic lobectomy (6,12,13). Therefore, our study aimed to investigate the effectiveness of c-PVI as compared with that of iv-PCA after thoracoscopic lobectomy for lung cancer.
Methods
This prospective randomized comparative study was approved by the Institutional Review Board and registered at clinicaltrials.gov (NCT01703351). Patients who were either diagnosed or suspected with early lung cancer (clinical stage T1–2aN0M0), who were fit to thoracoscopic lobectomy, and who signed the informed consent were eligible. Permission was obtained from every patient after providing detailed information about the study. After all trocars were inserted, the patient was randomly assigned to either the c-PVI or the iv-PCA group using computer-generated random numbers. Exclusion criteria included age younger than 18 years or older than 75 years, history of thoracic trauma or previous thoracic operation, chest pain for other reasons, severe pleural adhesion found at the time of operation, conversion to thoracotomy, and any complication that leads to intensive care unit admission. Chronic pain assessment was not conducted in patients who received repetitive chemical pleurodesis for air leakage, received adjuvant chemotherapy and/or radiation therapy, or refused the follow-up study after the operation.
Surgery
Thoracoscopic lobectomy with hilar and mediastinal lymph node dissection were performed with one utility incision and three ports. About 6 cm of utility incision was made at the 4th intercostal space (ICS) on the mid-axillary line level. A wound protector was applied without any rib spreader. The 12-mm trocars were placed at the 6th ICS on the anterior axillary line level and at the 7th ICS on the midaxillary line level. A 5-mm trocar was placed at the 5th ICS on the posterior axillary line level. Standard postoperative care was provided, and a chest tube was removed when there was no air leakage and the amount of drainage was less than 200 mL/day. Patients were discharged the day after tube removal.
Analgesia delivery
In the c-PVI group, a catheter with several side holes (On-Q system®; I-flow Corp, Lake Forest, CA, USA) was placed by a surgeon under thoracoscopic guidance at the end of operation. An introducer was inserted percutaneously 2 cm away from the vertebra on the 9th ICS and placed beneath the parietal pleura. The introducer was advanced up to the level of the 3rd ICS with caution to prevent any tearing on the parietal pleura. The introducer was removed after placing a catheter through it. After surgery, 0.5% ropivacaine was infused and maintained for 60 hours at a rate of 5 mL/hour. In the iv-PCA group, a patient-controlled infusion pump (Accufuser Plus, Wooyoung Medical Co., Ltd. Korea) was applied intravenously at the end of operation. It consisted of fentanyl 450–1,600 µg, nefopam 160 mg, and ramosetron 0.6 mg and was maintained for 60 hours at a rate of 5 mL/hour. Fentanyl dose was adjusted according to the patient’s body weight. When the patients felt severe pain, they were allowed to press the button to infuse 0.5 mL of the anesthetics.
In both groups, two tablets of Ultracet® (acetaminophen 650 mg plus tramadol 75 mg; Korea Janssen Pharmaceuticals, Inc., Seoul, Korea) were given orally three times a day. If the oral agent (Ultracet®) seemed ineffective in controlling postoperative pain, it was replaced by two capsules of Mypol® (codeine phosphate 10 mg plus ibuprofen 200 mg; Sungwon Adcock Pharm, Seoul, Korea). When a patient complained of pain despite the above-mentioned methods, 25 mg of intramuscular pethidine or 5 mg of oral oxycodone was provided. The oral agent was maintained for 2 weeks after discharge.
Acute pain assessment
Acute pain was assessed by a visual analogue scale (VAS; 0, no pain; 10, worst pain imaginable). VAS score was measured at rest in the evening of the operation day. Over the next 4 days, VAS score was recorded three times a day both at rest and on coughing. The highest VAS score of each day was used for further analysis and categorized as mild (VAS 0–2), moderate (VAS 3–5), and severe (VAS 6–10). Opioid dose, calculated as equivalent morphine dose (14), was also recorded during the first 4 days after operation. Pethidine 25 mg, fentanyl 1 mg, one capsule of Mypol®, and oxycodone 5 mg were substituted with morphine 3.3, 10, 0.8, and 10 mg, respectively.
Chronic pain and neuropathic pain assessment (quantitative sensory test)
Chronic pain was assessed by VAS at every follow-up. In addition, the item of “chest pain” in the quality of life (QoL) questionnaire was also used to identify mild chronic pain. Quantitative sensory testing (QST) was used to evaluate chronic neuropathic pain. QST is a non-invasive method for assessing peripheral nervous system disorder. We tested sensory detection threshold to touch, pressure, warmth, coolness as well as hot and cold pain at the outpatient clinic on 6, 12, and 24 weeks after surgery. Mapping of cool allodynia was also performed. The non-operated side forearm was tested first for reference, and then the non-operated side chest and operated chest were tested. On the operated chest, testing was done 2 cm anterior to the utility incision and at the chest tube site. Among the two values obtained from both sites, the more abnormal value was considered representative of the operated chest.
Tactile detection threshold and tactile pain threshold were assessed by Electronic Von Frey filament (IITC Inc., USA) using the method of levels (15). Thermal assessments were performed by the TSA-II-NeuroSensory Analyzer (Medoc Ltd., Israel) and included warm detection threshold (WDT), cool detection threshold (CDT), heat pain threshold (HPT), and cold pain threshold (CPT). A baseline temperature of 32 °C was applied; then, the temperature was changed at 1 °C per second. The temperature range was 0–50 °C. Finally, thermal sensitivities were tested among QST. Mapping of cool allodynia was performed around the surgical scar using a cool metal instrument at 10 °C. Mapping started from the outside and radially moved toward the scar at 2-cm intervals. Areas with cool allodynia were marked with a marking pen, and the marks were connected to form an outline of the outer margins. This line was traced onto a translucent sheet and scanned and analyzed using ImageJ (http://rsb.info.nih.gov/ij/).
QoL
QoL was assessed using the European Organization for Research and Treatment of Cancer quality of life questionnaire (EORTC QLQ-C30) and the lung cancer module (LC13). Every patient filled out the questionnaire before surgery and at 12 and 24 weeks after the operation. EORTC QLQ-C30 consisted of 30 items (6 functional scales and 9 symptom scales). LC13 consisted of 13 items (10 symptom scales). All scales were scored by the EORTC QLQ-C30 scoring manual (16), and the scores ranged from 0 to 100. A high score in global health status represents a high QoL. A high score in functional scale represents a high level of functioning. A high score in symptom scale represents a high level of symptoms or problems.
Statistics
To calculate the required sample size, we took into account the results of a previous study that had a similar clinical setting (17). A power analysis indicated that 41 patients were required in each group to detect a difference of 1 point in the VAS score [0–10] and 30-mg cumulative dose of opioids, with a type I error of 0.05 and power of 80%. A total of 96 patients were to be enrolled considering a 15% withdrawal rate.
The two groups were compared using Student’s t-test for continuous variables and Fisher’s exact tests for categorical variables. Continuous data are expressed as mean ± standard deviation, whereas categorical variables are expressed as counts and percentages. Tactile assessment data were analyzed using Mann-Whitney U test, whereas thermal assessment data and sensory mapping data were analyzed using Student’s t-tests. The results of the EORTC QLQ were grouped based on scales (global QoL, functional scales, symptom scales, and lung module function scales) and then compared with the Student’s t-test. A P value less than 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS version 21 statistical software program (SPSS Inc., Chicago, IL, USA).
Results
Eighty-four patients were enrolled in this study between October 2012 and August 2015. The study was closed earlier before the enrollment of 96 patients because of slow accrual. At the time of the operation, 5 patients were excluded from randomization due to severe pleural adhesion. Hence, only 79 patients were randomized to the c-PCA group (n=41) or to the iv-PCA group (n=38).
There were no significant differences in the baseline characteristics between the two groups (Table 1). All patients underwent the planned procedure by thoracoscopy, and there was no incidence of reoperation or other serious complications that required intensive care unit admission.
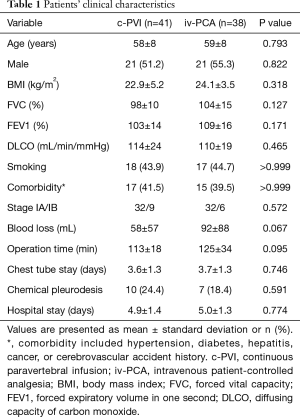
Full table
Acute pain assessment and amount of opioid used
Acute pain at rest or coughing was not significantly different between the two groups throughout 4 postoperative days (Table 2). Although the amount of additional opioids was not different between the two groups, the total amount of administered opioids was significantly lower in the c-PVI group than in the iv-PCA group when the fentanyl in the PCA pump was added in the calculation. The incidence of opioid-related side effects such as dizziness, constipation, pruritus, or nausea was similar in both groups (Table 3). However, the incidence of severe side effects leading to discontinuation of analgesics was significantly lower in the c-PVI group than in the iv-PCA group.
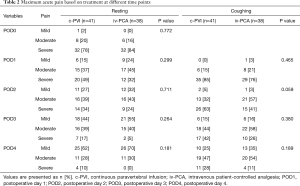
Full table

Full table
Assessment of chronic pain and QoL
During follow-up, 12 patients (c-PVI, n=7; iv-PCA, n=5) declined to continue joining the study. In 8 patients who received adjuvant chemotherapy for unexpected nodal metastasis (c-PVI, n=7; iv-PCA, n=1), assessment of chronic pain and QoL were not indicated. As a result, chronic pain and QoL could only be assessed in 59 patients (c-PVI, n=27; iv-PCA, n=32).
Chronic pain based on VAS score was reduced over time, and only 1 patient complained of chronic pain at the end of follow-up (24 weeks after surgery). However, when the EORTC QLQ-LC13 questionnaire was given, 25 patients (42%) answered that they felt mild pain in their response to “chest pain” item in the questionnaire (Table 4), although there was no statistical difference between the two groups.
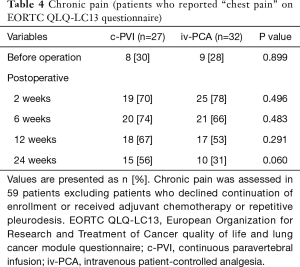
Full table
All patients completed the EORTC QLQ-C30-LC13 questionnaire during the follow-up period. No significant difference was observed between the two groups, but the global QoL was significantly higher in the PCA group at 12 weeks when broken down into separate scales (Table S1).
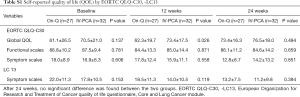
Full table
Neuropathic pain
The results of the tactile and thermal assessment are shown in Table S2 and Figure 1. On the contralateral forearm and non-operated chest, there were no significant differences in patients’ responses to tactile and thermal stimulation between the two groups. After operation, the thresholds for tactile and thermal stimulation significantly increased on the operated chest compared with those on the non-operated chest. The difference decreased over the course of time. However, no significant differences were observed between the two groups at each follow-up examination.
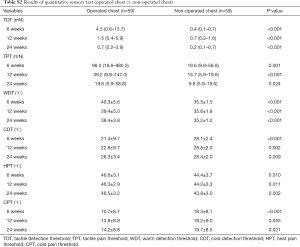
Full table
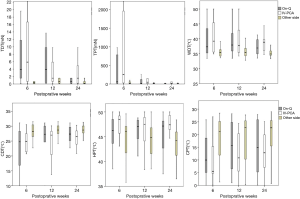
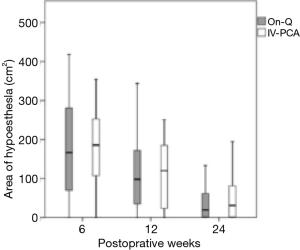
During sensory mapping, hypoesthesia to coolness was found in 54 (91.5%) patients at 6 weeks, 50 (84.7%) at 12 weeks, and 39 (66.1%) at 24 weeks. The total area of hypoesthesia showed no significant difference between the groups at each follow-up examination (Figure 2).
Post hoc analysis
We also performed post hoc analysis to investigate the characteristics of patients with chronic pain. Patients who reported chronic pain on the QoL questionnaire were compared with those who did not. Baseline patient characteristics were not significantly different between the two groups. Acute pain was significantly worse in the chronic pain group on postoperative day 3, at both resting and coughing states (Table 5). The results of QST did not show significant differences between the groups (Table 6).
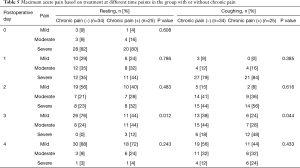
Full table
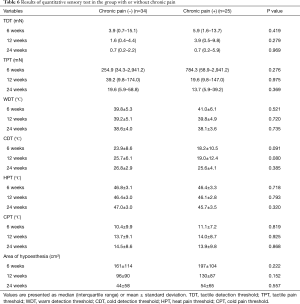
Full table
Discussion
Our study demonstrated that c-PVI of local anesthetics was as effective as iv-PCA in the management of acute and chronic pain while the total amount of opioid administration and the incidence of serious side effects were significantly reduced.
When comparing acute pain profiles between the two groups, the highest scores of pain at rest or coughing were used and divided into mild, moderate, and severe levels of pain, as generally accepted in previous research (18,19). This simple categorization is relevant in helping physicians make clinical decisions. For example, when patients continuously experience severe pain before discharge, oral analgesics could be increased. If this was insufficient, PCA should be reinitiated, with discharge delayed.
Although it has been well known that c-PVI can provide pain relief that is comparable to traditional iv-PCA with better side-effects profile after open thoracotomy (7-11), only a few existing studies have compared the analgesic effects of iv-PCA and c-PVI after thoracoscopic lobectomy (6,12,13). Similar to two reports from Asia (6,12), the current study demonstrated that c-PVI of local anesthetics could mitigate acute postoperative pain as effectively as iv-PCA. These results were different from those provided by another report (13), which showed a superiority in the analgesic effect of c-PVI using percutaneous technique. In our study, the infusion catheter was inserted beneath the parietal pleura under direct thoracoscopic vision. Although catheter insertion under magnified thoracoscopic guidance is more helpful, as compared to the percutaneous technique, there have been concerns about parietal pleura tearing, which could cause leakage of local anesthetics into the pleural cavity, leading to insufficient analgesic effect (6). Although the cases with severe pleural adhesion were excluded from our study, 24% of patients with mild to moderate pleural adhesion were enrolled in the c-PVI group. We speculated that there might be a higher chance of small pleural tearing during adhesiolysis, which could be a possible reason for the relatively higher VAS score in the c-PVI group.
This study showed that severe or repetitive side effects leading to the discontinuation of analgesic infusion were statistically lower in the c-PVI group. It is likely to stem from the difference in the amount of opioid administration. Given that opioid exposure can put patients at risk for long-term opioid use (20) or abuse (21), c-PVI could be a useful option to manage postoperative pain without increased risk of opioid abuse.
The incidence of chronic pain in this study is relatively higher compared with that (20–30%) reported in previous studies (1,22,23). The item of “chest pain” in the EORTC Q30 for QoL assessment, which has been associated with chronic post-surgical pain (24,25), was used to assess chronic pain in this study. There is a possibility that more patients with chronic pain could have been involved using the “chest item” item in EORTC Q30, since the questionnaire on the QoL represents any event of pain occurring over the past 1 week, whereas the numeric pain rating scale or VAS in other studies showed any pain during the past 24 hours. When comparing the chronic pain group with the no chronic pain group, there was no difference in baseline characteristics. Pain intensity at resting and coughing on postoperative day 3 when both c-PVI and iv-PCA were discontinued in most cases was highly associated with chronic pain. This result is similar with those in previous reports (22,25), which showed that pain intensity after thoracic operations correlated well with the occurrence of chronic pain. Therefore, we need to find the optimal pain management methods after discontinuation of c-PVI or iv-PCA to decrease the incidence of chronic chest pain.
Chronic neuropathic pain is common after thoracic surgery. Similar to other studies (26-28), each item in our QST showed increased thresholds on the operated side as compared to the control side in all patients. However, no difference in quantitative sensory item was found between the chronic pain group and the no chronic pain group, unlike in other studies that showed increased thermal thresholds after breast cancer (26) as well as hernia repair (27) or increased tactile detection threshold after thoracoscopic lobectomy (28). This discrepancy between chronic pain and chronic neuropathic pain profiles might stem from the fact that the QST in this study, which mainly reflected the change in the thresholds of the intercostal nerve, could not represent chronic pain after thoracoscopic lobectomy and lymph node dissection that could have occurred from visceral stimulation causing sensitization of the central nervous system, thus leading to the development of somatic chronic pain via viscero-somatic convergent mechanism (29,30). This finding is consistent with that of a previous study (31), which demonstrated that only a half of chronic pain after thoracic surgery showed a neuropathic component.
Study limitations
A major limitation of this study was the slow accrual rate and a study size smaller than anticipated. This weakened the analytic power of the study; therefore, subtle differences may not have been detected. We tried to evaluate the efficacy of c-PVI on various aspects. However, frequent visits and the time spent on conducting many neurological examinations were the major contributor to slow accrual and the decline during follow-up. Another limitation was the lack of confirmation on complete and accurate positioning of the c-PVI catheter because any catheter malpositioning or pleural leakage could affect the results. Nevertheless, this study is still meaningful in that it encompassed not only acute pain indices, but also chronic neuropathic pain and QoL, thereby attempting to evaluate comprehensively the efficacy of c-PVI compared with iv-PCA.
In conclusion, this clinical trial showed that c-PVI was similar to iv-PCA in terms of providing acute pain relief and reducing chronic neuropathic pain after thoracoscopic lobectomy, with reduction of the repetitive side effects of opioids.
Acknowledgements
Funding: This study was supported by a faculty research grant of Yonsei University College of Medicine (6-2012-0120).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Passlick B, Born C, Sienel W, et al. Incidence of chronic pain after minimal-invasive surgery for spontaneous pneumothorax. Eur J Cardiothorac Surg 2001;19:355-8. [Crossref] [PubMed]
- Hutter J, Miller K, Moritz E. Chronic sequels after thoracoscopic procedures for benign diseases. Eur J Cardiothorac Surg 2000;17:687-90. [Crossref] [PubMed]
- Liu CY, Lin CS, Liu CC. Asian perspectives in thoracic surgery: clinical innovation in Taiwan. J Thorac Dis 2016;8:S606-12. [Crossref] [PubMed]
- Maguire MF, Latter JA, Mahajan R, et al. A study exploring the role of intercostal nerve damage in chronic pain after thoracic surgery. Eur J Cardiothorac Surg 2006;29:873-9. [Crossref] [PubMed]
- Yie JC, Yang JT, Wu CY, et al. Patient-controlled analgesia (PCA) following video-assisted thoracoscopic lobectomy: comparison of epidural PCA and intravenous PCA. Acta Anaesthesiol Taiwan 2012;50:92-5. [Crossref] [PubMed]
- Jung J, Park SY, Haam S. Efficacy of subpleural continuous infusion of local anesthetics after thoracoscopic pulmonary resection for primary lung cancer compared to intravenous patient-controlled analgesia. J Thorac Dis 2016;8:1814-9. [Crossref] [PubMed]
- Gulbahar G, Kocer B, Muratli SN, et al. A comparison of epidural and paravertebral catheterisation techniques in post-thoracotomy pain management. Eur J Cardiothorac Surg 2010;37:467-72. [PubMed]
- Komatsu T, Sowa T, Takahashi K, et al. Paravertebral block as a promising analgesic modality for managing post-thoracotomy pain. Ann Thorac Cardiovasc Surg 2014;20:113-6. [Crossref] [PubMed]
- Kobayashi R, Mori S, Wakai K, et al. Paravertebral block via the surgical field versus epidural block for patients undergoing thoracotomy: a randomized clinical trial. Surg Today 2013;43:963-9. [Crossref] [PubMed]
- Baidya DK, Khanna P, Maitra S. Analgesic efficacy and safety of thoracic paravertebral and epidural analgesia for thoracic surgery: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg 2014;18:626-35. [Crossref] [PubMed]
- Ding X, Jin S, Niu X, et al. A comparison of the analgesia efficacy and side effects of paravertebral compared with epidural blockade for thoracotomy: an updated meta-analysis. PLoS One 2014;9. [Crossref] [PubMed]
- Hotta K, Endo T, Taira K, et al. Comparison of the analgesic effects of continuous extrapleural block and continuous epidural block after video-assisted thoracoscopic surgery. J Cardiothorac Vasc Anesth 2011;25:1009-13. [Crossref] [PubMed]
- Kosiński S, Fryźlewicz E, Wiłkojć M, et al. Comparison of continuous epidural block and continuous paravertebral block in postoperative analgaesia after video-assisted thoracoscopic surgery lobectomy: a randomised, non-inferiority trial. Anaesthesiol Intensive Ther 2016;48:280-7. [PubMed]
- Mercadante S, Caraceni A. Conversion ratios for opioid switching in the treatment of cancer pain: a systematic review. Palliat Med 2011;25:504-15. [Crossref] [PubMed]
- Walk D, Sehgal N, Moeller-Bertram T, et al. Quantitative sensory testing and mapping: a review of nonautomated quantitative methods for examination of the patient with neuropathic pain. Clin J Pain 2009;25:632-40. [Crossref] [PubMed]
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organisation for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. [Crossref] [PubMed]
- Wheatley GH 3rd, Rosenbaum DH, Paul MC, et al. Improved pain management outcomes with continuous infusion of a local anesthetic after thoracotomy. J Thorac Cardiovasc Surg 2005;130:464-8. [Crossref] [PubMed]
- Gerbershagen HJ, Rothaug J, Kalkman CJ, et al. Determination of moderate-to-severe postoperative pain on the numeric rating scale: a cut-off point analysis applying four different methods. Br J Anaesth 2011;107:619-26. [Crossref] [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med 2016;176:1286-93. [Crossref] [PubMed]
- Cicero TJ, Surratt H, Inciardi JA, et al. Relationship between therapeutic use and abuse of opioid analgesics in rural, suburban, and urban locations in the United States. Pharmacoepidemiol Drug Saf 2007;16:827-40. [Crossref] [PubMed]
- Bayman EO, Parekh KR, Keech J, et al. A prospective study of chronic pain after thoracic surgery. Anesthesiology 2017;126:938-51. [Crossref] [PubMed]
- Kwon ST, Zhao L, Reddy RM, et al. Evaluation of acute and chronic pain outcomes after robotic, video-assisted thoracoscopic surgery, or open anatomic pulmonary resection. J Thorac Cardiovasc Surg 2017;154:652-659.e1. [Crossref] [PubMed]
- Li M, Zhang M, Wang H, et al. The efficacy of Internet-based intervention on quality of life for patients with chronic post-surgical pain. Iran J Public Health 2016;45:1604-9. [PubMed]
- Yarnitsky D, Crispel Y, Eisenberg E, et al. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain 2008;138:22-8. [Crossref] [PubMed]
- Vilholm OJ, Cold S, Rasmussen L, et al. Sensory function and pain in a population of patients treated for breast cancer. Acta Anaesthesiol Scand 2009;53:800-6. [Crossref] [PubMed]
- Aasvang EK, Brandsborg B, Christensen B, et al. Neurophysiological characterization of postherniotomy pain. Pain 2008;137:173-81. [Crossref] [PubMed]
- Wildgaard K, Ringsted TK, Hansen HJ, et al. Quantitative sensory testing of persistent pain after video-assisted thoracic surgery lobectomy. Br J Anaesth 2012;108:126-33. [Crossref] [PubMed]
- Sarkar S, Hobson AR, Furlong PL, et al. Central neural mechanisms mediating human visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol 2001;281:G1196-202. [Crossref] [PubMed]
- Stawowy M, Funch-Jensen P, Arendt-Nielsen L, et al. Somatosensory changes in the referred pain area in patients with cholecystolithiasis. Eur J Gastroenterol Hepatol 2005;17:865-70. [Crossref] [PubMed]
- Steegers MA, Snik DM, Verhagen AF, et al. Only half of the chronic pain after thoracic surgery shows a neuropathic component. J Pain 2008;9:955-61. [Crossref] [PubMed]


