Diagnosis of thoracic endometriosis with immunohistochemistry
Introduction
Endometriosis is the growth and function of endometrial tissue outside of the uterine cavity. The presence of functional endometrium in the thoracic cavity is uncommon, and may cause hemoptysis in case of bronchial location or pneumothorax in the case of pleural or diaphragmatic involvement (1). Even if thoracic endometriosis is suspected based on an individual’s clinical background, it is difficult to identify confirmatory pathologic evidence of thoracic endometrial tissue. Classic histopathologic features of endometriosis are represented by the “triad” of endometrial glands, stroma, and hemosiderin-laden macrophages (2). However, the recognition of these elements is not always achieved on small tissue specimens. Ghigna et al. reported that this “triad” was recognized in 44% of the patients, and while only stroma was present in 56% of thoracic endometriosis cases (3). Since the pathological and immunohistochemical features of endometriosis in pleural or lung biopsy are not well understood, here we retrospectively discussed the clinical and pathological issues of 55 cases of pneumothorax in women. Our analyses led us to propose a novel strategy for the diagnosis of thoracic endometriosis in women who developed pneumothorax.
Methods
Institutional review board approval was obtained for this study, and the requirement for patient consent was waived off because of the retrospective nature of this study.
This study was a retrospective observation of 55 women aged from 8 to 62 years old that underwent surgery for pneumothorax from November 2001 through January 2018. Patients with a known history of underlying lung disease (chronic obstructive pulmonary disease, interstitial pneumonia, metastatic lung tumor) were excluded.
Data regarding a possible temporal relationship between pneumothorax episodes and menses were collected. We also recorded information about medical history, smoking habit, intraoperative findings, surgical technique, postoperative treatments, and recurrences after surgery.
For all cases, pathological specimens were reviewed by a pathologist. Immunohistochemistry was performed for all cases, using antibodies against ER, PR, and CD10. After deparaffinization, 4-µm-thick sections were treated with high pH type target retrieval solution (Target Retrieval Solution pH 9, Roche, Switzerland) for 40 min at 98 °C. The sections were then washed with buffer solution, and the primary antibodies were applied for 30 min. The antibodies used consisted of an anti-ER antibody (clone ER SP1, Roche), anti-PR antibody (clone PR 1E2, Roche), and anti-CD10 antibody (clone CD10 SP67, Roche). After washing the sections with buffer solution, a dextran polymer reagent conjugated with peroxidase and secondary antibodies was applied for 30 min. The sections were washed in water, and the samples were then counterstained with Mayer’s hematoxylin for 2 min. We also analyzed resected specimens of spontaneous pneumothorax in men who received surgery between January 2013 and November 2016 as a control. Immunohistochemical assessments of these specimens were also performed, and reviewed in the same way.
In all cases, procedures were performed by using video-assisted thoracoscopic surgery. After insertion of thoracoport, thoracoscopic exploration of the thoracic cavity was performed. We explored the thoracic cavity in the following sequence; whole parietal pleura, visceral pleura from apical portion, ventral portion, basal portion, dorsal portion to the interlobar portion of the lung, and diaphragm. When adhesions were observed, complete detachment was performed to allow whole exploration of the thoracic cavity. When bullae or blebs were found, they were resected with an endoscopic stapler, and the resection stump was covered with a polyglycolic acid sheet. All suspicious foci of endometriosis in both visceral and parietal pleura were resected. When diaphragmatic abnormalities such as perforation or brown nodules were identified, they were resected with an endoscopic stapler.
The statistical analysis was performed using SPSS statistical software (version 22, SPSS, Inc., Chicago, IL, USA). Recurrence rate was estimated using the Kaplan-Meier method, and dichotomous variables were compared using log rank test. Results were considered statistically significant for P<0.05.
Results
Clinical history
During the study period, 55 female patients underwent surgery for pneumothorax in our hospital. The mean age was 29.1 years (8–62 years old). Twenty-seven cases of pneumothorax (49.1%) occurred on the right side. Fifteen patients (27.3%) had smoking history. Pneumothorax occurring during menses was found in 12 patients (21.8%). All patients underwent surgery by video-assisted thoracoscopy. Among these patients, bullae or blebs were observed 51 individuals (92.7%), diaphragmatic abnormalities were observed in 5 patients (9.1%), and multiple brown visceral nodules were observed in 1 patient (1.8%). All diaphragmatic abnormalities and multiple brown visceral nodules were found on the right side. Fifty patients (90.1%) underwent only partial resection of the lung, 3 patients (5.5%) received partial resection of the lung and diaphragm, 1 patient (1.8) received only diaphragmatic resection, and 1 patient (1.8%) received biopsy of multiple visceral and diaphragmatic nodules (Table 1). One patient with multiple visceral and diaphragmatic endometriosis received pleurodesis immediately after surgery.
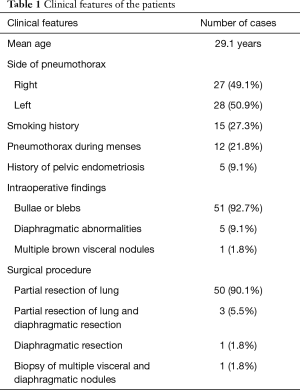
Full table
Pathological findings
Of all the 55 cases, only one contained both endometrial stroma and glands as identified by Hematoxylin-Eosin (HE) staining.
With regard to the immunohistochemistry findings, 37 cases (67.3%) exhibited positive staining for both ER and PR in stromal bland spindle cells of the lung. The remaining 18 cases (32.7%) were ER-, PR-, and CD10-negative. The ER- and PR-positive specimens, could be further classified according to two staining patterns, which we named ‘scattered’ and ‘aggregated’ pattern (Figure 1). Thirty-three cases (60.0%) were scattered pattern and four cases (containing the one case with endometrial stroma and glands) were aggregated pattern (Table 2). Scattered pattern samples were CD10-negative; in contrast, specimens with the aggregated pattern were CD10-positive. As a control, we stained resected specimens of 34 male cases with spontaneous pneumothorax with ER, PR, and CD10. Of these samples, 22 cases (65.7%) contained ER- and PR-positive bland spindle cells in the lung, and all had the scattered histological pattern (Table 2). Twelve cases (35.3%) were ER- and PR-negative. All cases were CD10-negative.

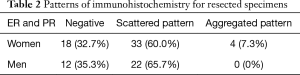
Full table
Our findings indicate that a diagnosis of thoracic endometriosis is relatively simple if endometrial stroma and glands are identified by HE staining. When endometrial glands are absent from the resected specimens, our second approach still permits a diagnosis of endometriosis if the “aggregated pattern” is confirmed by immunohistochemistry.
Outcome
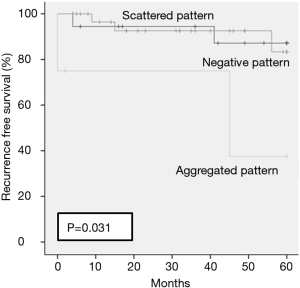
Mean follow-up period was 37.4 months. In all cases, pneumothorax recurred in 7 patients (12.7%) at 5 years. Recurrence rates at 5 years for the negative staining pattern, the scattered pattern, and the aggregated pattern were 12.8%, 16.7% and 62.5% respectively (P=0.031) (Figure 2). One patient with thoracic endometriosis received hormonal therapy after recurrence.
Discussion
The purpose of this study was to develop a method to diagnose thoracic endometriosis in women who developed pneumothorax. We were able to achieve this in samples with aggregation of bland spindle cells with or without glandular elements; the hormone-dependent nature of the cases was confirmed immunohistochemistry for ER, PR and CD10.
Classic histopathologic features of endometriosis are represented by a “triad” composed of endometrial glands, stroma, and hemosiderin-laden macrophages (2). However, in contrast to pelvic endometriosis, endometrial glands are not always present in thoracic endometriosis. Ghigna et al. reported that this “triad” was identified in 44% of cases, and stroma only (stromal endometriosis) was found in 56% of thoracic endometriosis (3). The diagnosis is always challenging when only small foci of endometrial stroma are found in lung tissue (3,4). Furthermore, sometimes it is difficult to distinguish endometrial stroma and inflammatory cells by HE staining. Given these challenges, diagnostic criteria based on immunohistochemistry are required for thoracic endometriosis. In our study, we could diagnose only one case as typical thoracic endometriosis by HE staining; this was from endometrial tissue that was resected from the chest wall. The other three cases of thoracic endometriosis were found in the subpleural connective tissue, and were diagnosed via immunohistochemical analysis using ER, PR, and CD10. These three cases might have been misdiagnosed as connective tissue or inflammatory cells if immunohistochemistry had not been performed.
In this study, we used immunohistochemistry of ER, PR, and CD10 to demonstrate the presence of endometrial cells. Thirty-three cases (60.0%) were stained by ER and PR in a scattered pattern, and four cases were stained by ER and PR CD10 in the aggregated pattern. Ultimately, we diagnosed four cases of aggregated pattern as endometriosis. Maria et al. also suggested that the diagnosis of endometrial tissue requires immunohistochemistry, including ER, PR, and CD10 as markers (3). Thoracic endometriosis frequently appears as nests of stromal cells; the recognition of these cells as endometrial of origin is difficult with routine histological examination. For this reason, pathologists should always evaluate the expression of the hormone receptors. One study of 84 cases of endometrial stroma and 21 cases of endometrial gland of the diaphragm that were analyzed with immunohistology reported that the frequencies of ER-, PR- and CD10-positivity in endometrial stroma were 88.1%, 100%, and 90.5%, respectively; in endometrial glands, the corresponding frequencies were 85.7%, 85.7%, and 0% (5). Taken together, these data suggest that ER-, PR-, and CD10-positive staining is necessary for identification of the endometrial stroma, whereas ER- and PR-positivity is required for identification of the endometrial gland.
We suggest that the staining pattern is necessary for precise diagnosis. The original point of our study was to use the resected specimens of spontaneous pneumothorax in men as control samples. We found that, even in men, many cases (61.8%) with stromal cell types exhibited positive staining for ER and PR in a scattered pattern. Therefore, resected specimens of pneumothorax in women may not be diagnosed as endometrial tissue if the stroma cells exhibited positive staining for ER and PR in a scattered pattern. In addition, the recurrence rate of pneumothorax with the scattered pattern was low (16.7% in 5 years) but with the aggregated pattern was high (62.5% in 5 years, P=0.031). Thus, from the standpoint of recurrence rates, a scattered pattern alone is insufficient for a diagnosis of thoracic endometriosis, whereas an aggregated pattern can be diagnosed as thoracic endometriosis with high risk for recurrence.
The main limitation of the present study was that our data were based on a relatively low number of cases. To support our current proposal, a greater number of female pneumothorax cases are required, and prospective studies that analyze the pathological and clinical findings are required. A second limitation was that despite the cases of thoracic endometriosis, endometrial tissue was not contained in the resected specimen incidentally. In the operation, we resected thoracic tissue from the lung, visceral and parietal pleura, and diaphragm, which was suspected as endometrial tissue. However, when clinical evidence and surgical evaluation support thoracic endometriosis, endometrial tissue is found in 30–50% of these cases (6).
Conclusions
Our study reveals that thoracic endometriosis is easy to diagnose when endometrial stroma and gland are present. In the case of stromal endometriosis, an “aggregated pattern” which stains positively for ER, PR, and CD10 might be necessary for accurate diagnosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Institutional review board approval was obtained for this study, and the requirement for patient consent was waived off because of the retrospective nature of this study.
References
- Joseph J, Sahn SA. Thoracic endometriosis syndrome: New observations from an analysis of 110 cases. Am J Med 1996;100:164-70. [Crossref] [PubMed]
- Flieder DB, Moran CA, Travis WD, et al. Pleuropulmonary endometriosis and pulmonary ectopic deciduosis: a clinicopathologic and immunohistochemical study of 10 cases with emphasis on diagnostic pitfalls. Hum Pathol 1998;29:1495-503. [Crossref] [PubMed]
- Ghigna MR, Mercier O, Mussot S, et al. Thoracic endometriosis: clinicopathologic updates and issues about 18 cases from a tertiary referring center. Ann Diagn Pathol 2015;19:320-5. [Crossref] [PubMed]
- Alifano M, Legras A, Jablonski CR, et al. Pneumothorax recurrence after surgery in women: clinicopathologic characteristics and management. Ann Thorac Surg 2011;92:322-6. [Crossref] [PubMed]
- Haga T, Kumasaka T, Kurihara M, et al. Immunohistochemical analysis of thoracic endometriosis. Pathol int 2013;63:429-34. [PubMed]
- Johnson MM. Catamenial pneumothorax and other thoracic manifestations of endometriosis. Clin Chest Med 2004;25:311-9. [Crossref] [PubMed]


