Effectiveness of neoadjuvant chemotherapy with etoposide and cisplatin followed by surgery for esophageal neuroendocrine carcinoma: a case report
Introduction
Patients with esophageal neuroendocrine carcinoma (ENEC), which is a rare disease, are considered to have a poor prognosis because of aggressive progression and widespread dissemination (1). The optimal treatment strategy for ENEC remains to be established. In this report, we describe a patient with ENEC who successfully achieved a pathologically complete response with neoadjuvant chemotherapy.
Case presentation
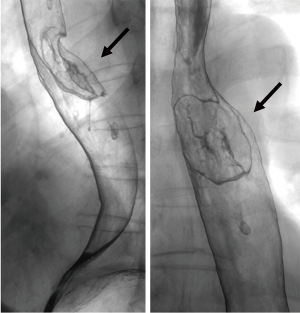
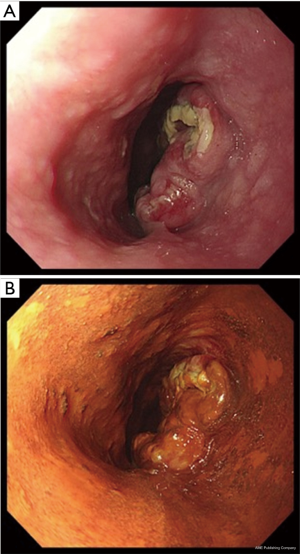
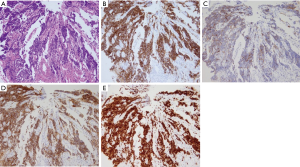
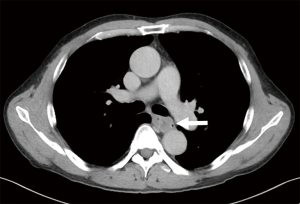
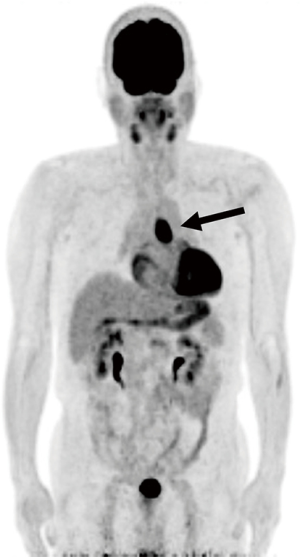
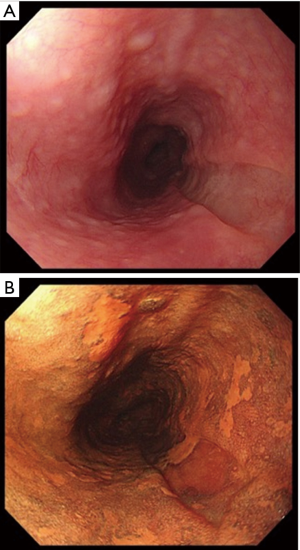
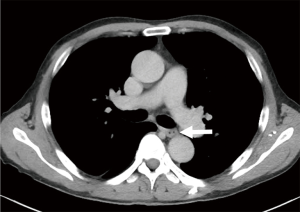
A 69-year-old man was referred to our hospital with a 1-month history of dysphagia. His medical history included atrial fibrillation and atrial flutter. No abnormalities were found on a physical examination. The laboratory findings showed mild renal dysfunction (serum creatinine level of 1.18 mg/dL; creatinine clearance 56.8 mL/min by Cockcroft-Gault equation), but the remaining findings were normal including the tumor marker levels (carcinoembryonic antigen, 2.5 ng/mL; carbohydrate antigen 19–9, 24.1 U/mL; squamous cell carcinoma antigen, 1.7 ng/mL; neuron-specific enolase, 11.7 ng/mL). A barium esophagogram revealed a 41-mm mass with ulceration (Figure 1). An esophagogastroduodenoscopy (EGD) revealed an ulcerated mass located at the posterior wall of the esophagus, 30 to 35 cm from the incisors (Figure 2). Pathological examination of an endoscopic biopsy specimen showed small round tumor cells with a high nuclear/cytoplasmic ratio (Figure 3A). Immunochemically, the cells were stained for CD56, chromogranin A and synaptophysin, and the MIB-1 index was more than 90% (Figure 3B,C,D,E). Computed tomography (CT) of the thorax revealed wall thickening in the middle thoracic esophagus (Figure 4). No evidence of lymph node or distant metastases was seen. Positron emission tomography (PET)-CT revealed the accumulation of fluorine-18-2-fluoro-2-deoxy-D-glucose (FDG) in the middle thoracic esophagus at a standard uptake value of 9.9 (Figure 5).
Thus, the patient received chemotherapy using etoposide (100 mg/m2, days 1–3) and cisplatin (80 mg/m2, day 1) under a diagnosis of ENEC (T3N0M0, stage IIA: TNM-UICC). We performed etoposide/cisplatin therapy according to the Japanese Clinical Oncology Group (JCOG) 1,213 protocol. The protocol proposes a dosage decrease of cisplatin if the patient’s creatinine level is more than 1.5 mg/dL. Because our patient’s creatinine level was 1.18 mg/dL, we performed chemotherapy using the standard dosage. However, since the patient’s creatinine clearance according to the Cockcroft-Gault equation was 56.8 mL/min, the patient also received sufficient hydration under careful observation. After the chemotherapy, no signs of a deterioration in renal function were seen.
The patient received one course of chemotherapy but developed grade 3 febrile neutropenia on day 10 (neutrophil count 15/µL) and grade 3 pneumonia on day 14 (according to the Common Terminology Criteria for Adverse Events, Version 4.0). He was treated with granulocyte colony-stimulating factor and antibiotics and had recovered by day 19.
After one course of chemotherapy, EGD revealed that the main tumor had markedly decreased in size and had changed to a clearly-demarcated, slightly depressed lesion (Figure 6); pathological examination of an endoscopic biopsy specimen showed no evidence of malignancy. A CT of the thorax revealed a reduction in the esophageal wall thickening (Figure 7). A PET-CT examination revealed no accumulation of FDG. The patient was diagnosed as having T1N0M0 stage I disease (TNM-UICC) after one course chemotherapy. Because the patient had developed febrile neutropenia accompanied by pneumonia, we felt that a second round of chemotherapy was not feasible.
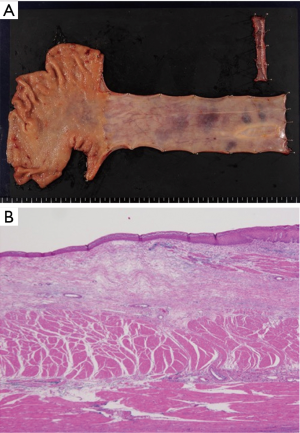
Seven weeks after the chemotherapy, a thoracoscopic esophagectomy with three-field lymph node dissection and gastric tube reconstruction through a posterior mediastinal route was performed. The patient was discharged from the hospital 20 days after the surgery with no complications. A histopathological examination of the resected specimen revealed no residual cancer cells (Figure 8). Lymph node metastasis was not observed. The patient is alive and has remained cancer-free for 2 years since the surgery.
Discussion
ENEC is an uncommon disease, accounting for 0.05–3.1% of all esophageal cancers, and the prognosis is extremely unfavorable (1). The median survival time (MST) is reportedly 3.4–19 months, and the 5-year survival rate is 6.7–15.4% (1). Although the use of surgery, chemotherapy, radiotherapy, and a combination of these therapies have been described, the optimal treatment strategy for ENEC remains to be established.
ENEC should be considered as a systemic disease because of its aggressive progression and widespread dissemination, and reports have recommended multimodal therapy based on chemotherapy (2). The MST of patients with distal metastasis (extensive disease: ED) treated using multimodality approaches is reportedly significantly longer than that of patients treated using a single method; similarly, the MST of patients with a main tumor with/without lymph node metastasis (limited disease: LD) treated using multimodality approaches was longer than that of patients treated using a single method, but the difference was not statistically significant (3).
Recently, the use of multimodal therapy, in which systemic chemotherapy is combined with local treatment, has been recommended for patients with ENEC (2,3); however, the optimal local treatment remains uncertain. Chen et al. summarized 40 cases of ENEC and reported that surgery and chemotherapy were independent prognostic factors for patients with ENEC in a multivariate analysis, and they recommended surgery combined with chemotherapy for the treatment of patients with LD of ENEC (4).
Although many reports of postoperative systemic chemotherapy for ENEC have been published, several studies have reported the use of neoadjuvant chemotherapy for ENEC. In resectable stage II/III esophageal squamous cell carcinoma (SCC), neoadjuvant chemotherapy with cisplatin plus 5-fluorouracil improves the survival time, compared with postoperative chemotherapy (Japanese Clinical Oncology Group; JCOG 9907) (5). Neoadjuvant chemotherapy is reportedly advantageous from three viewpoints: namely, the achievement of downstaging, a high complete resection rate, and a better completion of treatment, compared with postoperative chemotherapy (5). Although ENEC differs from SCC in terms of histological type and optimal chemotherapy regimens, neoadjuvant chemotherapy is expected to be effective for patients with ENEC.
To our knowledge, 9 cases of ENEC treated with neoadjuvant chemotherapy, including the present case, have been reported in the global medical literature (Table 1) (6-11). Because these reports were case reports, the possibility of a bias must be considered; however, the response rate was 89%, and two cases of long-term survival were reported. Thus, these reports suggested that neoadjuvant chemotherapy was an effectiveness treatment option for ENEC.

Full table
Regarding chemoradiotherapy, long-term survival after chemoradiotherapy treatment has been reported (1). However, Yau et al. summarized the non-operative management of ENEC and reported that chemoradiotherapy should not be recommended as the primary treatment for ENEC (12).
Although chemotherapy for small cell carcinoma of the lung is usually used as a reference when considering chemotherapy for ENEC, the optimum chemotherapy for ENEC remains to be established. Etoposide/cisplatin (EP) therapy is frequently used for ENEC. For patients with gastrointestinal neuroendocrine carcinoma treated with EP, the response rate was reported to be 75% (13). In the present case, severe febrile neutropenia occurred. Because grade 3 or 4 neutropenia reportedly occurs in 90% of patients treated with EP (14), careful observation is needed. In Japan, a randomized phase III study conducted by the JCOG for advanced neuroendocrine carcinoma of the digestive system comparing irinotecan/cisplatin therapy with EP therapy is currently in progress, and the results are expected in the near future. The establishment of optimal chemotherapy regimens and the investigation of neoadjuvant and adjuvant chemotherapy are needed for the treatment of ENEC.
Conclusions
We reported a patient with ENEC who achieved a pathological complete response after receiving neoadjuvant chemotherapy followed by surgery. Although ENEC is associated with a poor prognosis, neoadjuvant chemotherapy followed by an esophagectomy might be a potentially useful treatment option for patients with ENEC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Shinohara Y, Takeno S, Takahashi Y, et al. Successful chemoradiotherapy for small-cell carcinoma of the esophagus in an octogenarian japanese woman: report of the oldest case and review of long-term survival cases. Ann Thorac Cardiovasc Surg 2014;20:237-42. [Crossref] [PubMed]
- Lv J, Liang J, Wang J, et al. Primary small cell carcinoma of the esophagus. J Thorac Oncol 2008;3:1460-5. [Crossref] [PubMed]
- Zhu Y, Qiu B, Liu H, et al. Primary small cell carcinoma of the esophagus: review of 64 cases from a single institution. Dis Esophagus 2014;27:152-8. [Crossref] [PubMed]
- Chen SB, Yang JS, Yang WP, et al. Treatment and prognosis of limited disease primary small cell carcinoma of esophagus. Dis Esophagus 2011;24:114-9. [Crossref] [PubMed]
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68-74. [Crossref] [PubMed]
- Walker SJ, Steel A, Cullen MH, et al. Treatment of oesophageal small cell carcinoma by combined chemotherapy and surgical resection: Report of two cases and review of published cases. Thorax 1989;44:751-2. [Crossref] [PubMed]
- Muto I, Nishimaki T, Aizawa K, et al. Primary small cell carcinoma of the esophagus: Report of a case. Surg Today 1995;25:830-3. [Crossref] [PubMed]
- Nimura Y, Koide N, Nishio A, et al. Effective treatment with chemotherapy and surgical resection for small cell carcinoma of the esophagus: Report of a case. Hepatogastroenterology 1999;46:1778-81. [PubMed]
- Makino H, Tajiri T, Onda M, et al. Effectiveness of preoperative chemotherapy using carboplatin (CBDCA) and surgery against an esophageal small cell carcinoma. Dis Esophagus 2002;15:237-41. [Crossref] [PubMed]
- Koide N, Hiraguri M, Kishimoto K, et al. Small cell carcinoma of the esophagus with reference to alternating multiagent chemotherapy: Report of two cases. Surg Today 2003;33:294-8. [Crossref] [PubMed]
- Akiyama Y, Iwaya T, Shioi Y, et al. Effectiveness of neoadjuvant chemotherapy with cisplatin and irinotecan followed by surgery on small-cell carcinoma of the esophagus: A case report. Int J Surg Case Rep 2015;17:121-5. [Crossref] [PubMed]
- Yau KK, Siu WT, Wong DC, et al. Non-operative management of small cell carcinoma of esophagus. Dis Esophagus 2007;20:487-90. [Crossref] [PubMed]
- Yamaguchi T, Machida N, Morizane C, et al. Multicenter retrospective analysis of systemic chemotherapy for advanced neuroendocrine carcinoma of the digestive system. Cancer Sci 2014;105:1176-81. [Crossref] [PubMed]
- Iwasa S, Morizane C, Okusaka T, et al. Cisplatin and etoposide as first-line chemotherapy for poorly differentiated neuroendocrine carcinoma of the hepatobiliary tract and pancreas. Jpn J Clin Oncol 2010;40:313-8. [Crossref] [PubMed]