Effect of pneumoperitoneum and steep reverse-Trendelenburg position on mean systemic filling pressure, venous return, and microcirculation during esophagectomy
Introduction
Esophagectomy is a high-risk surgical procedure with high peri-operative morbidity (40%) and mortality (1%). The incidence of anastomotic leakage is high due to the fragile tissue perfusion of the gastric tube (1). Therefore, keeping adequate tissue perfusion during surgery is of utmost importance to decrease anastomotic leakage. During the procedure however, pneumoperitoneum is combined with steep reverse-Trendelenburg position to improve visibility in/to the surgical field. Pneumoperitoneum might cause unstable circulation like impaired cardiac function, increased mean blood pressure and central venous pressure (CVP) (2-6). These alterations in the macrocirculation can further impair microcirculatory perfusion.
The impact of pneumoperitoneum and reverse-Trendelenburg position on global haemodynamics is extensive. High intra-abdominal pressure (IAP) could lead to decreased venous return thereby impairing macrocirculation. Fluid infusion and vasopressors are employed to counteract high IAP aiming at maintaining stability of global circulation. However, the effect of a high venous pressure on microcirculatory perfusion is not well known during pneumoperitoneum and reverse-Trendelenburg. Pilot studies have shown that a high CVP was related to poor microcirculatory perfusion and increased morbidity in critically ill patients (7-9).
We hypothesise that a short-term increase in venous pressure is associated with poor sublingual microcirculatory perfusion during pneumoperitoneum with reverse-Trendelenburg in patients for minimally invasive esophagectomy. In the present observational study, we assessed global haemodynamics in relation to mean systemic filling pressure (MSFP) and venous return during pneumoperitoneum and steep reverse-Trendelenburg position; Furthermore, we investigated the relationship between macrocirculation and sublingual microcirculatory perfusion.
Methods
Patients undergoing minimally invasive esophagectomy were eligible. Patients with cardiac dysfunction (LVEF <40%), valve disease, and an allergy to colloids were excluded. The local ethical committee (Academic Medical Center, Amsterdam, The Netherlands) approved the protocol and the need to obtain written informed consent was waived (Academic Medical Center, registration number 2014_002). This observation pilot study was conducted according to the principles of the Declaration of Helsinki (version of Fortaleza, Brazil 2013) and in accordance with the Medical Research Involving Human Subjects Act (WMO).
Procedures
Induction of anesthesia
In the operating room, a thoracic epidural was placed at level Th5/6 or 6/7. General anesthesia was induced with propofol (approximately) 2–3 mg/kg, sufentanil for analgesia and 1.2 mg/kg. Rocuronium to facilitate intubation of the trachea. After induction, general anesthesia was maintained with sevoflurane at a minimal alveolar concentration of 1 MAC. An arterial line was inserted into the right or left radial artery. Next, a right subclavian tri-lumen central line and a double lumen gastric cannula were inserted. Fifteen minutes before incision 10 mL bupivacain 0.25% and 20 to 25 µg sufentanil was administered via the epidural catheter in two tempi. Forty-five minutes later a continuous infusion of bupivacain 0.25% was started at 6–8 mL/h. If epidural placement failed multimodal analgesic therapy was started, including ketamine 0.25 mg/kg and/or lidocaine 2% 6 mL/h.
Hemodynamic management during surgery
All oesophagectomies were treated according to a goal-directed hemodynamic fluid optimization protocol, which is standard of care in our institution. Following anaesthesia induction, when stable anaesthesia (epidural top up >15 min with adequate block) and hemodynamic conditions [mean arterial pressure (MAP) >65 mmHg or <20% from baseline MAP] were reached, the volume status of the patient was optimized by means of a fluid optimization protocol (Figure 1): One or more boluses of 250 mL tetraspan 6% were given to determine optimal stroke volume (SV). Optimal SV was defined as the SV that no longer increased by more than 10% after a fluid bolus. During the operation further colloid boluses were given only when the determined optimal SV declined more than 10% (the trigger SV). The baseline fluid maintenance (to correct for insensible loss) infusion was 1 mL/kg/h of crystalloids (sterofundin, Braun). Other haemodynamic goals were: MAP >65 mmHg or <20% from baseline MAP, heart rate (HR) <100 bpm. If necessary, norepinephrine (NE) infusion was used to achieve these goals. If SV was below the trigger SV and did not respond to a fluid bolus (without bleeding present), other causes for decreased SV were determined and treated.
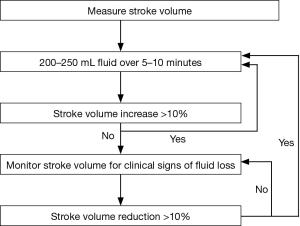
Moreover, the global hemodynamic parameters were monitored using EV1000 (Edwards, Irvine CA, USA) including: SV, SV variation (SVV), HR, diastolic blood pressure (DBP), systolic blood pressure (SBP) and MAP, CVP, cardiac output (CO). At every change in position the pressure reference was leveled to the tricuspid valve. In addition, pulse oximeter oxygen saturation (SpO2) and end-tidal CO2 (etCO2) were recorded.
Surgery and pneumoperitoneum
Patients underwent a (thoracic)laparoscopic transthoracic or transhiatal oesophageal resection and a gastric tube reconstruction with a cervical (transhiatal and three-stage transthoracic) or an intrathoracic (two-stage transthoracic) anastomosis. The choice for the surgical procedure depended on patient and tumor characteristics. The two-stage transthoracic approach started in the supine position with a laparoscopic gastric mobilization and an intraabdominal lymphadenectomy. For the second stage, the patient was turned into prone position and a thoracoscopic oesophageal mobilization and thoracic lymphadenectomy was performed with a subsequent intra thoracic anastomosis. With the three-stage transthoracic approach, the patient was turned into prone position after induction of anesthesia and the procedure started with a thoracoscopic oesophageal mobilization. During the second stage the patient was turned supine and a laparoscopy was performed. Finally, a left cervical incision was made for the anastomosis. During the transhiatal approach only laparoscopy was performed with a mediastinal oesophageal mobilization and lymphadenectomy via a widened hiatus.
Pneumoperitoneum was created with CO2 through a 12-mm supraumbilical camera trocar with a maximum IAP of 15 mmHg. During thoracoscopy a maximum of 6 mmHg intrathoracic pressure was used. Through three extra trocars (1 of 12 mm and 2 of 5 mm), 5–12-mm instruments were inserted into the peritoneal space. After the introduction of the trocars the patient was positioned in 20 degrees reverse-Trendelenburg (from a supine position in 0-degrees).
The timing of measurements
Intra-operative hemodynamic variables and sublingual microcirculation measurements were obtained [using sidestream dark-field (SDF) imaging at three different places] within 5 minutes after the following 3 time points:
- When supine, directly before the change to reverse-Trendelenburg position and the start of surgery (baseline, T =1);
- Prerequisites were: stable hemodynamic conditions; SV > trigger SV as defined by the fluid optimization protocol (Figure 1), MAP >65 mmHg or <20% difference from baseline arterial blood pressure (ABP) in case of pre-existent hypertension, epidural top up >15 minutes, and no recent change in NE dose or phenylephrine bolus (>5 minutes);
- After pneumoperitoneum insufflation (pneumoperitoneum, T =2);
- Directly after change to reverse-Trendelenburg position (pneumoperitoneum + reverse-Trendelenburg, T =3).
SDF imaging and analysis
The sublingual microcirculation was evaluated at three different spots for each timepoint using Sidestream Darkfield Imaging (MicroScan Video Microscope System; MicroVision Medical, Amsterdam, The Netherlands) (10,11). Three video clips of 20 seconds were recorded for each timepoint. Video clips for analysis underwent a quality-control test based on the quality of image resolution, clarity of image, and elimination of pressure-induced artifacts. The Automated Vascular Analysis (AVA version 3.0) software package (MicroVision Medical, Amsterdam, The Netherlands) was used for the analysis following the expert consensus conference (12). Both investigators were blinded to the macrocirculatory variables belonging to the images of microcirculatory flow. Expected changes of the microvascular flow index (MFI) were divided into four categories and each video was visually scored as zero as no flow, one as intermittent flow, two as slow flow (sluggish), and three as continuous flow. To evaluate interobserver variability, quantification of interobserver agreement was performed. Three observers (Y Ince, G Gruartmoner, DP Veelo) individually scored 11 different random clips of the microcirculation of 11 different patients.
Calculation of MSFP and related variables
- MSFP = (a*CVP) + (b*MAP) + (c*CO), where a and b are dimensionless constants (a + b =1, typically a =0.96, b =0.04), and c has the dimensions of resistance and is a function of the patient’s height, weight and age. The mathematical model of the systemic circulation is comprised of compliant arterial and venous compartments and resistances to blood flow. The model parameters are adjusted to match those of the patient’s current measured variables (13);
- Pressure gradient for venous return (PVR) = MSFP − CVP. The PVR was defined as the pressure difference between MSFP and CVP.
- Resistance of venous return (Resistance-vr) = (MSFP − CVP)/CO. The resistance downstream of MSFP was taken to reflect resistance for venous return and calculated as the ratio of the pressure difference between MSFP and CVP and CO.
Statistical analysis
A descriptive analysis was performed. All data were expressed as mean ± standard deviation, unless otherwise specified. Categorical variables are expressed as numbers and percentages. Comparisons of the related parameters according to the different time (T1 baseline, T2 pneumoperitoneum and T3 pneumoperitoneum + reverse-Trendelenburg) were performed using a General Linear Model Repeated Measures (GLMRM) (14,15). This model is an extension of the classical ANOVA, which allows handling both fixed effect (time points) and random effect (patient). GLMRM takes into account the correlation between multiple measurements of one patient and thus the estimated marginal means were adjusted for the covariates and the trends of the related parameters corresponding to the different time points could be shown. Paired data were compared with the t test or the Wilcoxon signed rank test. Comparisons of two continuous variables were performed using Spearman’s correlation. Moreover, all the pair measurement data of macrocirculation and microcirculation were divided into two groups according to the MFI value (group 1: MFI ≤2; group 2: MFI >2), and the difference was compared across groups. For the quantification of the interobserver agreement the number of agreements was noted. The weighted kappa between each of the observers was calculated. The threshold value of the receiver operating characteristic curves (ROC) for the variables used for predicting MFI ≤2.0 is the value corresponding with the highest average of sensitivity and specificity. All statistics were two-tailed, and a P value of less than 0.05 was considered to be significant. The statistical analysis was performed using the software package SPSS 13.0 (SPSS Inc., Chicago, IL, USA). The quantification of the interobserver agreement on SDF analysis was shown in Appendix 1.
Results
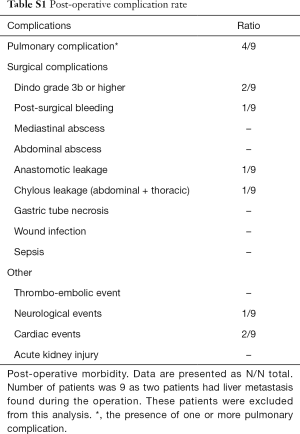
A total of 11 patients were enrolled, and 33 pairs of simultaneous recorded macrocirculatory and microcirculatory parameters were obtained. Patient and clinical characteristics are shown in Table 1. Six patients received a transthoracic (with most often a cervical anastomosis) and 5 a transhiatal approach. Twenty-seven percent (3/11) of patients had a medical history of hypertension, and 91% (10/11) patients received NE to maintain the blood pressure target during anesthesia and pneumoperitoneum. Moreover, two patients did not receive an epidural because of anatomic difficulties. Postoperative complications are found in Appendix 1, Table S1.

Full table
Full table
Evolution of macrocirculation and microcirculation at different points (T1, T2 and T3)
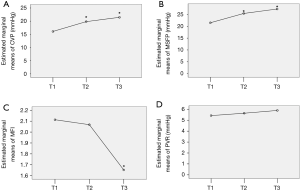
There was a significant change in CVP (P<0.0001), MSFP (P=0.001), SVV (P=0.005) and MFI (P=0.031) between T1, T2 and T3. However, the change in CO, PVR and MAP across different time points were not consistent and did not differ significantly (Table 2). Figure 2 shows the related variables CVP (Figure 2A), MSFP (Figure 2B), MFI (Figure 2C) and PVR (Figure 2D) at different time points. There was a significant higher CVP (Figure 2A) and MSFP (Figure 2B) and a lower MFI (Figure 2C) in T3 when compared to T1.

Full table
Relationship between macrocirculation and microcirculation
The MFI was significantly negatively correlated with CVP (r=−0.380, P=0.029) and MSFP (r=−0.367, P=0.0367), but there was no significant relationship of MFI and CO (r=−0.024, P=0.893), and MFI and MAP (r=−0.254, P=0.154).
Prediction of MFI ≤2
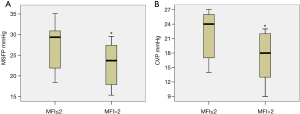
There were 13 measurements of sublingual microcirculation with MFI ≤2.0 and 20 measurements with MFI >2.0. Measurements with a MFI ≤2.0 were associated with a significant higher CVP and MSFP compared to measurements with a MFI >2.0 (CVP, 17.4±4.8 vs. 21.8±4.9, P=0.009; MSFP, 23±5 vs. 28±6, P=0.013, Figure 3).
The cutoff value and areas under the ROC for the variables used for predicting MFI ≤2.0 are shown in Table 3 and Figure 4. CVP and MSFP are associated with a larger area under the ROC to predict MFI ≤2 than CO and MAP. A cutoff value of 23 mmHg for CVP resulted in a specificity of 100% and a sensitivity of 61.54% to detect a MFI <2.
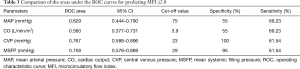
Full table
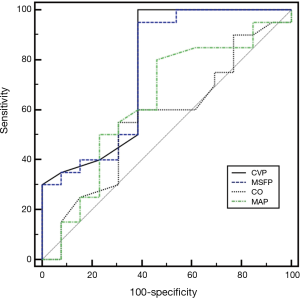
Discussion
Adequate tissue perfusion during surgery is of utmost importance to decrease complications such as anastomotic leakage. In this study we investigated the association of macro- and microcirculatory perfusion in surgical patients in which pneumoperitoneum and steep reverse-Trendelenburg position were employed. The main findings of our study were that the increase in CVP and MSFP are associated with poor microcirculatory perfusion even when MAP and venous return on the macrocirculatory level have been maintained. Secondly, using a CVP ≥23 mmHg resulted in a sensitivity of 61.54% and a specificity of 100% to detect a MFI ≤2.
We found a significant increase in CVP and MSFP resulting from the pneumoperitoneum. Moreover, there was no change in pressure gradient for venous return, the resistance to venous return and CO when compared to baseline. Several factors should be taken into account when interpreting these results. (I) The stress response from the pneumoperitoneum and the use of NE could contribute to the restoration of the pressure gradient for venous return when compared to baseline. Maas et al. showed that NE could increase the pressure gradient for venous return and CO in postoperative cardiac surgery patients (16); (II) in our study, fluid status was optimized before pneumoperitoneum and position changes, and should have provided sufficient intravascular volume for transferring unstressed volume to stressed volume when NE was used. Interestingly, we found that the venous return at the level of macrocirculation could be restored during pneumoperitoneum with fluids or vasopressors, while the microcirculatory perfusion was still impaired. Moreover, we found a progressive increase in CVP during pneumoperitoneum despite reverse-Trendelenburg positioning. This is partly in contrast to an earlier study from Odeberg and colleagues (17), who found that increased CVP during pneumoperitoneum decreased when patients were turned to the reverse-Trendelenburg position. One possible explanation for the difference between the studies is the use of our “optimized fluid administration protocol” (SV targets: no longer increased by more than 10% after a fluid bolus) to maintain the patients volume status before pneumoperitoneum and position changes. This may have decreased the effect of the reverse-Trendelenburg maneuver on filling pressures; (III) most of our patients had an epidural catheter in place and therefore vasodilatory effects due to local anesthetic-induced sympathicolysis might have aggravated the influence of pneumoperitoneum on resistance to venous return. In contrast, epidural anesthesia has been shown to improve gastric microcirculation in open esophagectomy, but this effect might be different during the endoscopic approach (18).
Interestingly, we found MFI to be significantly associated with CVP but not with a change in CO and MAP. Limited variation of CO and MAP during the operation might explain the lacking relationship between MFI and CO/MAP. In our study, a decrease in MAP of more than 20% at T3 was seen in only three patients, while CVP was at a relatively high level already at the baseline. Therefore, we inferred an over-high CVP was independently related to poor microcirculatory perfusion even when MAP and macrocirculatory venous return had been restored during the operation. Our study supports the findings of Vellinga and colleagues (7) who showed a decrease in MFI with higher CVP independent of perfusion pressure (MAP) in septic patients. The authors found CVP >12 mmHg to be the only significant predictor for a capillary MFI <2.6 in a multivariate model.
Because the studies on sublingual MFI during esophagectomy are very limited in number, it remains difficult to determine the optimal value of MFI with regard to the outcome (anastomotic leakage). A threshold for MFI of 2.6 is the lower limit of the 95% confidence interval for healthy subjects (19). A value of MFI ≤2 indicated a no-flow or intermittent or sluggish microcirculatory flow in the observed tissue area (1), which reflects a critically impaired tissue perfusion (severe ischemia) (1,12). Because the impairment of microcirculatory perfusion might be only temporarily during pneumoperitoneum, a threshold of MFI ≤2 would be more relevant in the intraoperative setting. Hence, this threshold as an indicator of poor microcirculatory flow perfusion seems reasonable in our study. Further studies are required to validate the relationship of MFI ≤2 and anastomotic leakage during esophagectomy. We found that a CVP ≥23 mmHg was able to detect an MFI ≤2 with a sensitivity of 61.54% and a specificity of 100%. Therefore, an over-high CVP value may be taken as an indicator of poor microcirculatory perfusion during pneumoperitoneum. Of note, whether a temporary severe impaired microcirculatory perfusion during pneumoperitoneum might influence organ function or anastomotic healing is uncertain but will be clinically relevant particularly for patients undergoing esophagectomy. In theory, an increased CVP after pneumoperitoneum may aggravate venous congestion at the level of the gastric tube, especially during the McKeown operation, where the anastomosis is made during the abdominal phase and directly after pneumoperitoneum. An experimental study in pigs showed that venous congestion in the splanchnic tissues due to reduced venous return aggravates a decrease in blood flow in the gastric tube after termination of arterial blood supply. The impaired microvascular perfusion can partly be counteracted by application of nitroglycerin (20). Hence, more attention should be paid the potential detrimental effects of a high CVP on organ function or anastomotic healing. We postulate that hemodynamic evaluation should include both the macro- and microcirculation.
There are several limitations of this study. First, the sample size was small as only 11 patients were enrolled. In our opinion, the sample size of the study may not be optimal, but should be sufficient to draw a conclusion that may guide clinical practice. Second, it is uncertain whether we can assume that local gastric microcirculation shows the same pattern as the sublingual circulation and can be used interchangeably to guide haemodynamic therapy during these procedures. Indeed, one recent study showed a temporary dispersion between gastro-intestinal microcirculation and sublingual circulation during severe shock in septic patients (21). Although derangements in microcirculation have been suggested to be a predictor of outcome in some critically ill populations (22,23), the use of microcirculation based therapeutic models has not been widely implemented, especially not in the perioperative setting. In addition, it is unclear how long these derangements should exist to influence outcome. In this study we did not follow up on MFI and therefore do not know whether the changes in MFI aggravate or resolute over time, especially during the making of the anastomosis, or its influence on outcome. Further studies need to be performed to answer those questions. Third, the cardiac function was not evaluated in our study. The potential impairment in cardiac function due to pneumoperitoneum might cause a high CVP and MSFP. In addition, in four patients measurements took place after thoracoscopy had been completed, so theoretically this could have influenced cardiac function because of dissection of mediastinal lymph nodes and sacrifice of nerves. Fourth, there might be a limitation of the mathematical coupling in related data (CVP, MAP, CO and MSFP) since MSFP was derived from a calculated formula in our study. Moreover, one study showed a bias (−6.0+3.1 mmHg) and disagreement (−12.4 and 0.3 mmHg) between MSFP calculated from the analogue model and MSFP derived from inspiratory hold manoeuvers (24). However, the changes in MSFP and PVR calculated from the analogue model were consistent with the cardiovascular model described by Guyton during a fluid challenge (25,26). Changes in MSFP calculated from the analogue model accurately tracked MSFP derived from inspiratory hold manoeuvers in an animal model (27). Hence, the limitation of using the analogue measurement technique does most likely not affect our conclusion. Further studies are required to show the effect of medical interventions (fluid infusion or vasopressors) on venous return and MSFP during pneumoperitoneum and reverse-Trendelenburg position by continuously measuring MSFP over a longer period of time.
Conclusions
During pneumoperitoneum and reverse-Trendelenburg positioning, both high CVP and MSFP are associated with a low sublingual microcirculatory perfusion even when macrocirculation is restored. An high CVP was related to the poor microcirculatory flow perfusion during the pneumoperitoneum of operation. The venous return at the level of macrocirculation could be restored during pneumoperitoneum with fluids and NE, while the microcirculatory perfusion was still impaired.
Appendix 1 Validation of the operating room microcirculatory flow score
For validation of the scoring system we compared the interobserver agreement between the three different observers. Observer number 1 was considered to be the reference because it was the investigator with more experience in microcirculatory imaging acquisition technique with sidestream dark field (SDF). Agreement of observers number 2 and 3 compared to observer number 1 is described below:
Agreement observer 1 and 2
Number of observed agreements was 11 (91.67% of the observations). Number of agreements expected by chance was 4.8 (40.28% of the observations). Kappa =0.860 [standard error (SE) of kappa =0.134; 95% confidence interval: 0.598 to 1.000]. The strength of agreement is considered to be “very good”.
Agreement observer 1 and 3
Number of observed agreements was 10 (83.33% of the observations). Number of agreements expected by chance: 5.0 (41.67% of the observations). Kappa =0.714 (SE of kappa =0.180), 95% confidence interval: 0.361 to 1.000). The strength of agreement is considered to be “good”. The weighted kappa is 0.750. The strength of agreement is considered to be “good”.
Acknowledgements
Funding: H He received a fund from China Scholarship Council (No. 201608110082) and the Organization Department of Beijing Municipal Committee (No. 2015000020124G072).
Footnote
Conflicts of Interest: DP Veelo and MW Hollmann have received support [contract was made, under name: quality improvement project, Academic Medical Center (AMC)] from Edwards regarding a different project but for the same patient population. Funders were not involved in any activities regarding this study. DP Veelo, SS Gisbertz and MI van Berge Henegouwen have done consultancy work for Edwards in light of a different project. C Ince has developed SDF imaging and is listed as inventor on related patents commercialized by MicroVision Medical (MVM) under a license from the AMC. He has been a consultant for MVM in the past, but has not been involved with this company for more than 5 years now, except that he still holds shares. The other authors have no conflicts of interest to declare.
Ethical Statement: The local ethical committee (Academic Medical Center, Amsterdam, The Netherlands) approved the protocol and the need to obtain written informed consent was waived (Academic Medical Center, registration number 2014_002).
References
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Alfonsi P, Vieillard-Baron A, Coggia M, et al. Cardiac function during intraperitoneal CO2 insufflation for aortic surgery: a transesophageal echocardiographic study. Anesth Analg 2006;102:1304-10. [Crossref] [PubMed]
- Rademaker BM, Odoom JA, de Wit LT, et al. Haemodynamic effects of pneumoperitoneum for laparoscopic surgery: a comparison of CO2 with N2O insufflation. Eur J Anaesthesiol 1994;11:301-6. [PubMed]
- Popescu WM, Bell R, Duffy AJ, et al. A pilot study of patients with clinically severe obesity undergoing laparoscopic surgery: evidence for impaired cardiac performance. J Cardiothorac Vasc Anesth 2011;25:943-9. [Crossref] [PubMed]
- Youssef MA, Al Mulhim A. Physiologic effects of pneumoperitoneum in adults with sickle cell disease undergoing laparoscopic cholecystectomy (a case control study). Surg Endosc 2008;22:1513-8. [Crossref] [PubMed]
- Veelo DP, Geerts BF. Anaesthesia during oesophagectomy. J Thorac Dis 2017;9:S705-12. [Crossref] [PubMed]
- Vellinga NA, Ince C, Boerma EC. Elevated central venous pressure is associated with impairment of microcirculatory blood flow in sepsis: a hypothesis generating post hoc analysis. BMC Anesthesiol 2013;13:17. [Crossref] [PubMed]
- Long Y, Su L, Zhang Q, et al. Elevated Mean Airway Pressure and Central Venous Pressure in the First Day of Mechanical Ventilation Indicated Poor Outcome. Crit Care Med 2017;45:e485-92. [Crossref] [PubMed]
- Pilcher DV, Scheinkestel CD, Snell GI, et al. High central venous pressure is associated with prolonged mechanical ventilation and increased mortality after lung transplantation. J Thorac Cardiovasc Surg. 2005;129:912-8. [Crossref] [PubMed]
- Milstein DM, Bezemer R, Ince C. Sidestream Dark‐Field (SDF) Video Microscopy for Clinical Imaging of the Microcirculation. In: Leahy MJ. editor. Microcirculation imaging. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2012:37-52.
- Goedhart PT, Khalilzada M, Bezemer R, et al. Sidestream dark-field (SDF) imaging: a novel stroboscopic LED ring-based imaging modality for clinical assessment of the microcirculation. Opt Express 2007;15:15101-14. [Crossref] [PubMed]
- De Backer D, Hollenberg S, Boerma C, et al. How to evaluate the microcirculation: report of a round table conference. Crit Care 2007;11:R101. [Crossref] [PubMed]
- Parkin WG, Leaning MS. Therapeutic control of the circulation. J Clin Monit Comput 2008;22:391-400. [Crossref] [PubMed]
- Roy A. Estimating correlation coefficient between two variables with repeated observations using mixed effects model. Biom J 2006;48:286-301. [Crossref] [PubMed]
- McCulloch CE, Searle SR. Generalized, Linear, and Mixed Models. John Wiley and Sons, 2000.
- Maas JJ, Pinsky MR, de Wilde RB, et al. Cardiac output response to norepinephrine in postoperative cardiac surgery patients: interpretation with venous return and cardiac function curves. Crit Care Med 2013;41:143-50. [Crossref] [PubMed]
- Odeberg S, Ljungqvist O, Svenberg T, et al. Haemodynamic effects of pneumoperitoneum and the influence of posture during anaesthesia for laparoscopic surgery. Acta Anaesthesiol Scand 1994;38:276-83. [Crossref] [PubMed]
- Al-Rawi OY, Pennefather SH, Page RD, et al. The effect of thoracic epidural bupivacaine and an intravenous adrenaline infusion on gastric tube blood flow during esophagectomy. Anesth Analg 2008;106:884-7. [Crossref] [PubMed]
- Edul VS, Enrico C, Laviolle B, et al. Quantitative assessment of the microcirculation in healthy volunteers and in patients with septic shock. Crit Care Med 2012;40:1443-8. [Crossref] [PubMed]
- Buise MP, Ince C, Tilanus HW, et al. The effect of nitroglycerin on microvascular perfusion and oxygenation during gastric tube reconstruction. Anesth Analg 2005;100:1107-11. [Crossref] [PubMed]
- Boerma EC, van der Voort PH, Spronk PE, et al. Relationship between sublingual and intestinal microcirculatory perfusion in patients with abdominal sepsis. Crit Care Med 2007;35:1055-60. [Crossref] [PubMed]
- Benes J, Giglio M, Brienza N, et al. The effects of goal directed fluid therapy based on dynamic parameters on post-surgical outcome: a meta-analysis of randomized controlled trials. Crit Care 2014;18:584. [Crossref] [PubMed]
- Trzeciak S, Dellinger RP, Parrillo JE, et al. Microcirculatory Alterations in Resuscitation and Shock Investigators. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med 2007;49:88-98. [Crossref] [PubMed]
- Maas JJ, Pinsky MR, Geerts BF, et al. Estimation of mean systemic filling pressure in postoperative cardiac surgery patients with three methods. Intensive Care Med 2012;38:1452-60. [Crossref] [PubMed]
- Cecconi M, Aya HD, Geisen M, et al. Changes in the mean systemic filling pressure during a fluid challenge in postsurgical intensive care patients. Intensive Care Med 2013;39:1299-305. [Crossref] [PubMed]
- Gupta K, Sondergaard S, Parkin G, et al. Applying mean systemic filling pressure to assess the response to fluid boluses in cardiac post-surgical patients. Intensive Care Med 2015;41:265-72. [Crossref] [PubMed]
- Lee JM, Ogundele O, Pike F, et al. Effect of acute endotoxemia on analog estimates of mean systemic pressure. J Crit Care 2013;28:880.e9-15. [Crossref] [PubMed]


