Uniportal video-assisted thoracoscopic surgery lobectomy and segmentectomy for pulmonary sequestration
Introduction
Pulmonary sequestration is a nonfunctioning lung mass with an abnormal connection to tracheobronchial trees and an anomalous systemic artery supply. It accounts for a small portion of pulmonary malformations (1,2). There are two subtypes of pulmonary sequestration, namely intralobar sequestration (ILS) and extralobar sequestration (ELS). ILS shares the visceral pleura with the lobe in which it lies whereas ELS develops its own visceral pleurae. Pulmonary sequestration could be silent with patients and diagnosed as adults (3). Adult patients usually manifest upper airway syndrome, hemoptysis, or repeated lung infections. Diagnosis is usually confirmed by imaging results based on the above symptoms. Surgical resection of the diseased lung, either lobar or sublobar resection, has been established as the definitive treatment for eliminating active symptoms and preventing the progression of airway complications; it can be carried out safely using video-assisted thoracic surgery (VATS) (4-6). Uniportal VATS is challenging for surgeons. The literature on uniportal VATS anatomical resection for treating pulmonary sequestration is limited. Herein, we evaluate the perioperative results and potential advantages of uniportal VATS anatomical resection for managing pulmonary sequestration and compare the perioperative outcomes to those obtained with the multiportal VATS approach.
Methods
We reviewed our surgery database and found 19 patients who had undergone VATS resection for pulmonary sequestration in the period July, 2007, to September, 2017. We categorized the patients into uniportal VATS and multiportal VATS groups. Demographics (age and gender), pulmonary lung function, characteristics of pulmonary sequestration (location, subtype, origin of systemic artery), and perioperative data (operation time, blood loss, complications, duration of pleural drainage, and duration of postoperative hospital stay) were recorded for all patients. A comparison of baseline data and perioperative outcomes was made between the uniportal and multiportal VATS groups and between patients with different extent of resections. This study was approved by the Institutional Review Board of the National Cheng Kung University Hospital (B-ER-105-390).
Surgical technique
All patients were operated on under general anesthesia with selective one-lung ventilation using a double-lumen endotracheal tube or single-lumen endotracheal tube with an endobronchial blocker. The patients were positioned in the full lateral decubitus position and the surgeon always stood at the ventral site of the patient.
In the uniportal approach, a 3- to 5-cm utility port was created in the 4th or 5th intercostal space (ICS) at the anterior axillary line. In the multiportal approach, two additional 1.5-cm ports were created for the thoracoscope (6th or 7th ICS, mid-axillary line) and assistance (5th or 6th ICS, posterior axillary line). An XS-sized Alexis wound protector (Applied Medical, Rancho Santa Margarita, CA, USA) was applied at the utility port. A 30˚ 10-mm thoracoscope was used for vision.
The systemic feeding artery was the first structure dissected out. It was divided using Endo-GIA (Covidien, Mansfield, MA, USA) or Endo-cutter (Ethicon Endo-Surgery, Guaynabo, Puerto Rico, USA) at the beginning of the operation. Although the exact location of the systemic feeding artery was determined based on preoperative computed tomography (CT) or magnetic resonance imaging, mostly it could be identified around the pulmonary ligament and embedded in adhesive tissue.
The extent of parenchymal resection depended on how extensive the pulmonary sequestration was. The most common resection was standard lobectomy; wedge resection and segmentectomy were reserved for small and localized disease. Stapling method (Endo-GIA or Endo-cutter for parenchyma, bronchus and vessels) was used to carry out segmentectomy and fissure fusion to minimize air leakage from lung parenchyma.
Each specimen was placed in a self-sterilized plastic zipper storage bag and pulled out. One chest tube in uniportal VATS and two chest tubes in multiportal VATS were placed for pleural drainage. The chest tube was removed based on the drainage amount and status of air leakage of the lung.
Statistical analysis
For the clinical data, the continuous variables are presented as means ± standard deviations and the categorical variables are presented as frequencies (%). Differences between uniportal VATS and multiportal VATS groups were determined using the Mann-Whitney U test for continuous variables and the Chi-square test and Fisher’s exact test for categorical variables. A P value of less than 0.05 was considered to indicate a significant difference. IBM SPSS Statistics for Windows, version 19.0 (IBM Corp., Armonk, NY, USA), was used for analysis.
Results
A total of 19 cases (8 males and 11 females; 17 ILS and 2 ELS) were analyzed. The mean age was 38.8 years (range, 17–62 years). There were 17 ILS and 2 ELS. All ILS were located at lower lobes, 13 on the left side and 4 on the right side. The two ELS were between the lower lobe and the diaphragm. Because of the sharing of a common pulmonary artery and a pulmonary vein, the two ELS were resected along with the adjacent lower lobe (one left lower lobe and one right lower lobe) after the aberrant systemic artery was divided. No patient presented an obstructive pattern in the lung function test before surgery. Seven patients underwent uniportal VATS resection and 12 patients underwent multiportal VATS resection. The mean age and gender distributions were not significantly different between groups. No intraoperative conversion, surgical mortality or major complications were identified among the patients. Postoperative complications included one prolonged air leakage and one chylothorax, which led to longer pleural drainage and postoperative hospital stay. Systemic artery identification and ligation were completed in all cases except for that without a systemic artery. In some patients that had certain respiratory symptoms, such as hemoptysis and repeated pneumonia, they were all free from symptoms after surgery.
A comparison of uniportal and multiportal VATS groups is given in Table 1. We only conducted anatomical resection for pulmonary sequestration after 2016, and there was no wedge resection in the uniportal VATS group. Therefore, the type of surgical resection reached statistically significant difference between the two groups (P=0.033). The mean surgery time and blood loss were 152 min and 64 mL in the uniportal VATS group, respectively; these values were not significantly different from those in the multiportal VATS group (174 min, P=0.526; 88 mL, P=0.652). The pleural drainage duration and postoperative hospital stay were also similar between the two groups (3.0 vs. 4.0 d, P=0.171; 4.7 vs. 5.8 d, P=0.514, respectively).
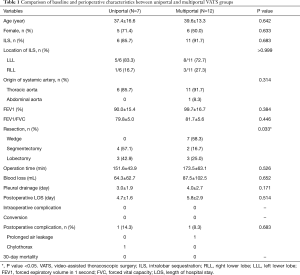
Full table
Six segmentectomies were performed: 5 basal segmentectomy (S7+8+9+10) (3 in uniportal VATS and 2 in multiportal VATS) and 1 left S6+9+10 segmentectomy (uniportal VATS) (Figure 1). The operation time and blood loss amount in wedge resection, segmentectomy, and lobectomy were not significantly different (Table 2). The duration of pleural drainage and post-operative hospital stay were not significantly different between the three types of resection.

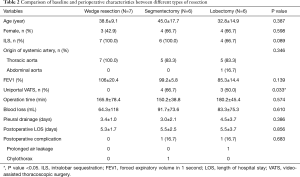
Full table
There was one left-lower-lobe primary lung cancer, lymphoepithelioma-like carcinoma, coexisting with a left extralobar pulmonary sequestration; both were resected in uniportal VATS.
Discussion
Pulmonary sequestration is an uncommon congenital anomaly with a reported incidence rate of 0.15–1.8% (1,2). Asymptomatic sequestration would be neglected in the patient’s growth. In adults, pulmonary sequestration is diagnosed after a patient’s complain of symptoms or when having a health check. In this study, we excluded children and infants, for whom surgical intervention was usually conducted using open thoracotomy. For the studied patients, all lesions were confined in the lower lung (ILS) or between the lower lung and the diaphragm (ELS), which is consistent with the most common distribution of pulmonary sequestration (1).
Surgical resection of the diseased lung is recommended for symptomatic patients and should also be performed for some asymptomatic patients to control and prevent recurrent pulmonary infection by stopping the inflammatory process and to lower the incidence of airway complications (2,3).
The feasibility and safety of VATS lobectomy for pulmonary sequestration have been investigated (5,6,8). A comparison between VATS and open surgery for managing this congenital anomaly showed that VATS was not inferior to open surgery and that it might have some advantage, such as less blood loss and shorter postoperative hospital stay (9-11). The reported conversion rates of VATS lobectomy ranged from 0% to 18.8% (6,10,11). The most common cause of conversion was intraoperative injury to the aberrant systemic artery, followed by post-infectious adhesion (5,9-11). Identification and control of the systemic artery were regarded as the most crucial steps in the operation of pulmonary sequestration. Failure to control the systemic artery, especially when it derives from the abdominal aorta, may lead to disastrous results (12). The aberrant systemic artery was usually located within the pulmonary ligament and embedded in thickened adhesive tissue after repeated pulmonary infection. A careful dissection of the pulmonary ligament, a bit at a time and as close to the descending aorta as possible, was done to avoid injury to the aberrant systemic artery, and sometimes, its branches. In our series, all aberrant systemic arteries were identified and controlled without any major bleeding. Branches of the aberrant systemic artery identified in the operation which were different from preoperative prediction did not influence the surgical methods.
For minimally invasive surgery, some surgeon attempt to reduce the number of access ports for managing pulmonary sequestration. Gonzalez-Rivas reported successful management of two cases of ILS using double-port VATS lobectomy (13). Uniportal VATS lobectomy has been applied to both ELS (14,15) and ILS (16-18). Recently, Li et al. published a series of 110 adult cases with pulmonary sequestration managing thoracoscopically and there were 8 uniportal VATS lobectomy reported (11). Although they did not describe the subgroups of uniportal VATS separately, no significant difference in perioperative outcomes was identified between the 1–2 ports and 3–4 ports subgroups. In this study, we compared uniportal VATS with multiportal VATS directly. Our data show that uniportal VATS is comparable to multiportal VATS in terms of blood loss, surgical time and postoperative outcomes. The relief of postoperative symptoms and recovery for the uniportal group are encouraging.
For the most common location of pulmonary sequestration, we recommend the 5th intercostal utility incision in uniportal approach for direct line of sight and to facilitate lower lung dissection. As illustrated in Figure 2, the pulmonary ligament and the aberrant systemic artery from the descending aorta can be approached easily, and the stapler can be applied without difficulty. Pleural adhesion is a great concern in VATS for pulmonary sequestration. We have treated infectious pulmonary disease with VATS anatomical resection for a decade and have published a couple of studies on VATS for pulmonary tuberculosis (19,20). In comparison to the extensive and tenacious adhesion at the upper pleural space in pulmonary tuberculosis, it is much easier to approach and dissect the relatively benign adhesion in pulmonary sequestration from the suggested utility port.
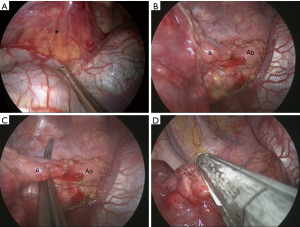
Wedge resection is an alternative modality for small and peripheral ILS (21,22). A series reported by Lin et al. showed that VATS wedge resection had a shorter operation time and less blood loss compared to those for lobectomy (22). However, there is doubt whether the preserved parenchyma contains the diseased part after wedge resection (23). We performed 7 VATS wedge resections in the multiportal group before 2016. The surgery time and blood loss of wedge resection were not significantly better than those for segmentectomy or lobectomy since there was no difference in perioperative variables. Atypical segmentectomy, such as left S6+9+10 segmentectomy was conducted using uniportal VATS in our series. We still have to find and control the aberrant systemic artery and the drainage vein of the diseased lung in wedge resection for ILS. In addition, defining the affected territory of ILS and performing an adequate wedge resection without compromising normal pulmonary vessels are usually time-consuming. We can decide which segment is totally unaffected from CT images. We go directly for segmentectomy, either conventional or atypical, in order to preserve the healthy portion of the lung. Since we had completed more than 500 VATS segmentectomies, including 250 uniportal surgeries, in our institute (unpublished data), anatomical segmentectomy may be an easy and suitable procedure for removing a localized ILS and preserving more healthy lung parenchyma. We do not routinely have patients undergoing pulmonary function test after operation; actual difference in recovery of pulmonary function between different extents of pulmonary resections was unknown in our series. It is believed that segmentectomy provided mild postoperative and small long-term benefits over lobectomy in pulmonary function (24).
Although we demonstrated that uniportal surgical lung resection is a feasible method for the treatment of lung sequestration, we considered a relatively small number of cases. Moreover, this was a retrospective single-institution study. This is an uncommon disease and patients may not receive treatment if they present no symptoms. More surgeries and perioperative results are required to confirm our analysis.
In conclusion, although uniportal VATS pulmonary sequestration resection is challenging, this surgical approach is safe and feasible. We presented a series of uniportal VATS anatomical resections for pulmonary sequestration with satisfactory perioperative results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board of the National Cheng Kung University Hospital (B-ER-105-390).
References
- Savic B, Birtel FJ, Tholen W, et al. Lung sequestration: report of seven cases and review of 540 published cases. Thorax 1979;34:96-101. [Crossref] [PubMed]
- Corbett HJ, Humphrey GM. Pulmonary sequestration. Paediatr Respir Rev 2004;5:59-68. [Crossref] [PubMed]
- Van Raemdonck D, De Boeck K, Devlieger H, et al. Pulmonary sequestration: a comparison between pediatric and adult patients. Eur J Cardiothorac Surg 2001;19:388-95. [Crossref] [PubMed]
- Wan IY, Lee TW, Sihoe AD, et al. Video-assisted thoracic surgery lobectomy for pulmonary sequestration. Ann Thorac Surg 2002;73:639-40. [Crossref] [PubMed]
- Kestenholz PB, Schneiter D, Hillinger S, et al. Thoracoscopic treatment of pulmonary sequestration. Eur J Cardiothorac Surg 2006;29:815-8. [Crossref] [PubMed]
- Shen JF, Zhang XX, Li SB, et al. Complete video-assisted thoracoscopic surgery for pulmonary sequestration. J Thorac Dis 2013;5:31-5. [PubMed]
- Lin TH, Huang WL, Chang CC, et al. Uniportal VATS left S segmentectomy for ILS at left superior segment (S). Asvide 2018;5:577. Available online: http://www.asvide.com/article/view/25549
- Tsang FH, Chung SS, Sihoe AD. Video-assisted thoracic surgery for bronchopulmonary sequestration. Interact Cardiovasc Thorac Surg 2006;5:424-6. [Crossref] [PubMed]
- Liu C, Pu Q, Ma L, et al. Video-assisted thoracic surgery for pulmonary sequestration compared with posterolateral thoracotomy. J Thorac Cardiovasc Surg 2013;146:557-61. [Crossref] [PubMed]
- Wang LM, Cao JL, Hu J. Video-assisted thoracic surgery for pulmonary sequestration: a safe alternative procedure. J Thorac Dis 2016;8:31-6. [PubMed]
- Li Q, Xie D, Sihoe A, et al. Video-assisted thoracic surgery is associated with better short-term outcomes than open thoracotomy in adult patients with intralobar pulmonary sequestration. Interact Cardiovasc Thorac Surg 2018;26:284-7. [Crossref] [PubMed]
- Li R, Li H, Liang T, et al. Undiagnosed pulmonary sequestration results in an unexplained hemorrhagic shock in thoracoscopic pulmonary lobectomy. J Clin Anesth 2016;35:485-7. [Crossref] [PubMed]
- Gonzalez D, Garcia J, Fieira E, et al. Video-assisted thoracoscopic lobectomy in the treatment of intralobar pulmonary sequestration. Interact Cardiovasc Thorac Surg 2011;12:77-9. [Crossref] [PubMed]
- Tamura M, Shimizu Y, Hashizume Y. Single-incision video-assisted thoracic resection for extrapulmonary sequestration: a case report. J Cardiothorac Surg 2014;9:22. [Crossref] [PubMed]
- Halezeroğlu S, Okur E, Ergene G. Single-Incision Video-Assisted Thoracic Surgery for an Extralobar Sequestration in a Child. Innovations (Phila) 2016;11:64-6. [Crossref] [PubMed]
- Lin ZW, Xu ST, Wang Q. Uniportal video-assisted thoracic lobectomy in a semiprone position for the treatment of a large intralobar pulmonary sequestration. Interact Cardiovasc Thorac Surg 2015;21:542-4. [Crossref] [PubMed]
- Sihoe ADL, Luo Q, Shao G, et al. Uniportal thoracoscopic lobectomy for intralobar pulmonary sequestration. J Cardiothorac Surg 2016;11:27. [Crossref] [PubMed]
- Kim CW, Kim DH. Single-incision video-assisted thoracic surgery lobectomy in the treatment of adult communicating bronchopulmonary foregut malformation with large aberrant artery. J Thorac Dis 2016;8:E148-51. [PubMed]
- Yen YT, Wu MH, Lai WW, et al. The role of video-assisted thoracoscopic surgery in therapeutic lung resection for pulmonary tuberculosis. Ann Thorac Surg 2013;95:257-63. [Crossref] [PubMed]
- Tseng YL, Chang JM, Liu YS, et al. The Role of Video-Assisted Thoracoscopic Therapeutic Resection for Medically Failed Pulmonary Tuberculosis. Medicine (Baltimore) 2016;95. [Crossref] [PubMed]
- Sakuma T, Sugita M, Sagawa M, et al. Video-assisted thoracoscopic wedge resection for pulmonary sequestration. Ann Thorac Surg 2004;78:1844-5. [Crossref] [PubMed]
- Lin ZW, Gu J, Xu ST, et al. Video-Assisted Thoracoscopic Surgery for Intralobar Pulmonary Sequestration: Wedge Resection Is Feasible in Limited Peripheral Lesions. Thorac Cardiovasc Surg 2016;64:456-60. [Crossref] [PubMed]
- Nakanishi R, Iwanami T. Video-assisted thoracic surgery superior segment-sparing lower lobectomy for intralobar pulmonary sequestration. J Laparoendosc Adv Surg Tech A 2008;18:290-2. [Crossref] [PubMed]
- Charloux A, Quoix E. Lung segmentectomy: does it offer a real functional benefit over lobectomy? Eur Respir Rev 2017;26. [Crossref] [PubMed]


