Hemodynamic changes lead to alterations in aortic diameters and may challenge further stent graft sizing in acute aortic syndrome
Introduction
Thoracic endovascular aortic repair (TEVAR) offers an effective treatment for several aortic pathologies, including emergency situations in acute aortic syndrome (1-3). Accurate sizing of the proximal landing zone is an essential requirement to avoid stent migration, occlusion, or endoleak resulting in re-interventions (4).
One potential pitfall in stent graft sizing may arise from shifted intravascular fluid balance during blood loss and the inflammatory nature of the acute aortic syndrome. Previous studies already demonstrated that sizing of aortic diameters (ADs) in patients undergoing TEVAR is not based on a static model. Already in balanced hemodynamic conditions, heartbeat-dependent morphologic changes can occur (5,6). Thus, an increasing number of protocols arise, including those of computed tomography (CT) and magnetic resonance imaging techniques, taking possible dynamic changes into account for a more precise stent graft sizing (7,8).
Patients with acute aortic syndrome are frequently admitted due to significant blood loss or hypovolemic shock, and the challenge of trapping aortic dynamics multiplies with the addition of impaired hemodynamic conditions. Blood loss or hypovolemic shock may dramatically decrease ADs (9). As CT is mostly performed at the time of diagnosis, a timely delay often occurs in definite TEVAR involving further changes in hemodynamic conditions. Above that, an impaired CT quality in emergency cases might be another reason for the difficulties in stent graft sizing. Especially CTs from emergency patients that were referred from outside facilities often lack electrocardiograph (ECG)-tracing and special aortic protocols incorporating aortic dynamics. Considering a necessary early treatment, further CT diagnostics would lead to therapeutic delay and doubled contrast agent administration in patients with often already impaired kidney function.
Intravascular ultrasound (IVUS) can contribute useful information regarding intraluminal real-time sizing and provide important assessment of underlying diseases (10); however, its significance in acute aortic syndromes has not been evaluated so far.
This study aimed to determine the effect of changes on the AD in emergency TEVAR before and after hemodynamic stabilization and the potential benefits of IVUS-guided stent graft sizing after stabilized hemodynamic conditions in acute aortic syndromes. As most important benchmark, we focused on the AD at the level distal to the left subclavian artery (LSA) as a frequent landing zone in TEVAR.
Methods
Study design
We performed a single-center, retrospective study analyzing the results of CT and IVUS measurements at the LSA level in patients with aortic syndrome.
All patients who underwent CT and IVUS for diagnostics or interventional treatment of the thoracic aorta between October 2006 and August 2016 were examined. Patients were basically divided on the basis of the shock index (= heart rate/systolic blood pressure) into two groups. The patients who showed stabile hemodynamics (shock index <1) and underwent IVUS for diagnostic purposes only to exclude a significant aortic disease, further conservative treatment, or planned TEVAR electively were categorized into the elective group. Conversely, patients with a shock index >1 who underwent IVUS in emergency cases with subsequent TEVAR after admission for clinical purposes (<24 hours initial diagnosis) were categorized into the emergency group. Basic hemodynamic parameters were analyzed at time of admission and time of IVUS procedure.
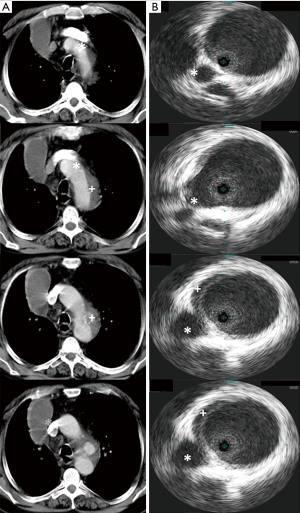
ADs were measured at the level distal to the LSA using CT at time of diagnosis as a benchmark for the measurements of the further landing zone (Figure 1). IVUS measurements were obtained from the IVUS procedure with following TEVAR depending on the underlying diagnosis. The minimum and maximum diameters were measured and ECG-traced in both IVUS and CT to obtain the mean diameters. All patients were diagnosed and further treated at our institution (major tertiary referral center) by a team of interventional cardiologists, cardiothoracic surgeons, and anesthesiologists with significant expertise in acute aortic syndrome. An experienced cardiologist who was unaware of the details of the present study performed the IVUS and the following TEVAR, if necessary.
The study was approved by the local ethics committee and was conducted in accordance with the Declaration of Helsinki. Patient records were de-identified and analyzed anonymously. Therefore, the local ethics committee approved the retrospective analysis of the patient data without the need to obtain patient consent.
IVUS
IVUS was performed using the Visions PV 0.035 catheter system (Volcano, San Diego, CA, USA). The IVUS catheter used a 10-MHz frequency ultrasound and had a maximum imaging diameter of 60 mm. A pigtail catheter was inserted into the ascending aorta. Over a long guidewire that was introduced over the catheter, the IVUS catheter was positioned in the ascending aorta close to the aortic valve. The IVUS probe was manually retracted until the distal part of the sheath. The grayscale images were simultaneously collected digitally. Gyrating movements were used in an attempt to obtain an optimal cross-sectional aortic image. The cardiologist who performed the IVUS had a previous experience in over 50 IVUS measurements of the aorta.
CT
CT was either performed using a 64-row multidetector scanner (Somatom Definition; Siemens Healthcare, Forchheim, Germany) or already conducted at the referring outside facility. The following examination protocol was referred to our institution. Continuous scans covering the entire aorta, including the proximal supraoptic vessels down to the groin, were conducted. An iodinated contrast agent (120 to 140 mL) was continuously injected into the right antecubital vein using an 18-G catheter at an infusion rate of 3.5 mL/s. To ensure maximum contrast concentration in the aorta, a circular region of interest (ROI) was placed in the ascending aorta. As soon as the signal intensity in the ROI reached a threshold of 120 Hounsfield units, the patients were instructed to maintain an inspiration breath hold, at which point data acquisition commenced. A second late arterial phase scan was performed after a delay of 15 seconds, covering the same area.
Statistical analysis
Continuous variables were presented as means ± standard deviations (SD) and categorical variables as numbers and percentages. The Mann-Whitney U test was used for the comparison of categorical variables. The Student’s t-test was used for the analysis of continuous variables. Variables were compared using the unpaired t-test. Two-tailed tests were used for all analyses. Values of P<0.05 were considered statistically significant. All data were analyzed and statistical analyses were performed using the SPSS 24 (Chicago, IL, USA) for Mac and Microsoft Excel 2011 for Mac.
Results
Study population
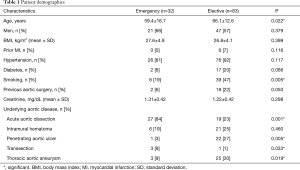
A total of 115 patients underwent CT and IVUS of the thoracic aorta between October 2006 and August 2016 for different clinical purposes. Eighty-three patients underwent elective CT and IVUS; 70 of them required TEVAR due to underlying aortic pathology, which was planned electively (84%). The percentage of implanted stents in the emergency group (n=32) was similar (27 patients, 84%). The indications for treatment included aortic rupture with hemothorax and/or hemomediastinum, persistent pain, refractory hypertension, acute limb, intestinal, or renal ischemia, a maximum descending or thoracic AD of 40 mm (acute aortic dissection) or 50 mm (chronic aortic dissection).
The reasons for non-implantation in the emergency group were immediate surgery with aortic replacement (n=4) and indicated immediate treatment but restricted life expectancy (n=1).
One patient was excluded owing to non-confirmation of the expected underlying disease. The relevant patient demographics are shown in Table 1.
Full table
Basic characterization of the hemodynamic conditions in both groups
The hemodynamic status of the patients was measured by assessing the basic clinical variables, including the hematocrit level, heart rate, and blood pressure at time of admission and at time of later IVUS-procedure. Notably, the time of admission did not equal the time of CT. Twenty-six CT studies (81%) were already done at outside facilities yielding to the primary diagnosis acute aortic syndrome. No hemodynamic records at time of CT were available.
The emergency patients showed severe compromised hemodynamic status at time of admission, whereas the elective group had consistently normal to high range blood pressures. After hemodynamic compensation blood pressure and heart rate did not show differences in both groups at time of following IVUS-procedure, but use of peri-interventional noradrenaline use was higher in the emergency group.
All patients, including elective and emergency, had post-TEVAR monitoring at our ICU for at least 24 hours. Important hemodynamic data are shown in Table 2.

Full table
Comparison of the CT and IVUS measurements
The emergency group showed significant smaller diameters at the level of the LSA at time of CT compared to the following IVUS measurements. The tendency was the same, regardless of whether the minimum or mean diameter in IVUS was used (CT: 26.9±4.1 mm, IVUSmean: 32.0±4.0 mm; IVUSmin: 30.8±4.2 mm; P=0.001). In contrast, in the elective group no differences were found between CT and IVUS measurements at time of CT and following IVUS (CT: 31.3±4.4 mm, IVUSmean: 31.7±4.1 mm; IVUSmin: 31.0±4.2 mm; P=0.065, Figure 2).
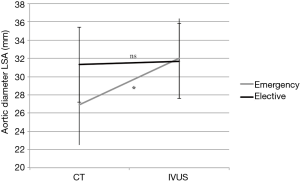
Comparing the mean differences between CT and IVUS we found a significant increase in AD from time of CT to following IVUS in the emergency group. This finding applies also to the minimum and mean IVUS-diameter (Table 3).

Full table
Discussion
Our results highlight the relationship between IVUS and CT assessments of the AD in normal and impaired hemodynamic conditions and present limitations in stent graft sizing to consider in emergency situations. Our results were in line with previous data regarding the accuracy of IVUS assessment in patients with acute aortic syndrome (10); however, no further differentiation was conducted in emergency patients with positive shock index to date.
Stent graft sizing for TEVAR, especially in the aortic arch, can be challenged by the overestimation of the aortic luminal diameter in IVUS owing to off-center measurements and non-tangential views (11,12), but precise sizing of the stent graft is essential to avoid further complications and re-interventions. Taking this into account, measuring the minimum AD at the level distal to the LSA has already been shown to provide a good correlation with CT measurements (10).
In comparison, at the level distal to the LSA we found consistent ADs between CT and later IVUS in the elective group, whereas the emergency group had significant decreased AD in CT before hemodynamic stabilization. The lower blood pressure and hematocrit level, and elevated heart rate support the presumption of impaired hemodynamic conditions at time of admission indicating a loss of intravascular volume.
Previous studies have shown the clinical dilemma regarding stent graft sizing in trauma patients with altered hemodynamic conditions (9,13). The normal pulsatility of the aorta already shows a variation of ~18% for the ascending and descending thoracic aorta, and the current CT protocols further consider the dynamic aspect of aortic evaluation (14,15). Shrinking of the AD due to hypovolemic conditions has already been shown in a porcine model. The induced blood loss led to ~40% decrease in the AD measured by IVUS (16). However, hypovolemic conditions do not only decrease ADs via impaired intravascular filling; the neurohumoral response to hypotension also leads to vasoconstriction through the angiotensin II-mediated pathway and increases further loss of diameter (17). In our study, we only used basic clinical parameters, such as heart rate, blood pressure and hematocrit level, as markers for intravascular filling; additional influences of the aortic wall are not the subject of the current study and need further evaluation.
Typically, CT is performed at the time of diagnosis and often with a timely delay between CT and TEVAR. This may result in possible underestimation of the stent graft, since differences in the measured diameters can affect stent graft sizing due to changes in hemodynamic and volume filling, e.g., in patients with aortic rupture the associated hemodynamic compromise can affect intravascular volume (18).
IVUS enables real-time assessment from intra-luminal and therefore turns out to be a feasible and valuable alternative approach to CT in aortic diseases. Especially in cases of impaired imaging quality of previous CT studies, IVUS might provide relevant information regarding diameter assessment.
Moreover, aortic CT studies are complex; in particular, CT studies from outside facilities with low experience are challenging when it comes to stent graft sizing (18). IVUS assessment in these cases can be useful without major timely delays when definite TEVAR is needed; however, its use in emergency TEVAR underlies some limitations.
Further studies are needed to evaluate the long-term outcome of these patients regarding potential endoleak and aortic remodeling.
One major limitation is the timely gap between the CT from outside facilities and the assessment of hemodynamic parameters at time of admission. Moreover, comparisons have to be drawn carefully due to the different underlying pathologies in both collectives.
Conclusions
Although CT is a state-of-the-art method for stent graft sizing in TEVAR, IVUS is a valuable alternative approach during emergency situations. Impaired hemodynamic conditions in acute aortic syndrome at time of diagnosis might lead to impaired ADs in CT assessment and therefore challenge precise stent graft sizing. IVUS might provide useful real-time information adjusting the actual hemodynamic situation and therefore may be considered in emergency situations to allow for a careful stent graft selection and limit further stent graft related complications.
Acknowledgements
We acknowledge support by the Open Access Publication Fund of the University of Duisburg-Essen.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the local ethics committee (17-7761-BO) and written informed consent was waived.
References
- Wiedemann D, Mahr S, Vadehra A, et al. Thoracic endovascular aortic repair in 300 patients: long-term results. Ann Thorac Surg 2013;95:1577-83. [Crossref] [PubMed]
- Brunkwall J. Thoracic endovascular repair--this is just the beginning. J Cardiovasc Surg (Torino) 2006;47:485-6. [PubMed]
- Nienaber CA, Kische S, Ince H, et al. Thoracic endovascular aneurysm repair for complicated type B aortic dissection. J Vasc Surg 2011;54:1529-33. [Crossref] [PubMed]
- Resch T. Failure modes and secondary endovascular interventions after endovascular aortic repair. J Cardiovasc Surg (Torino) 2017;58:218-27. [PubMed]
- Laskowski I, Verhagen HJ, Gagne PJ, et al. Current state of dynamic imaging in endovascular aortic aneurysm repair. J Endovasc Ther 2007;14:807-12. [Crossref] [PubMed]
- van Keulen JW, van Prehn J, Prokop M, et al. Dynamics of the aorta before and after endovascular aneurysm repair: a systematic review. Eur J Vasc Endovasc Surg 2009;38:586-96. [Crossref] [PubMed]
- Ko JP, Brandman S, Stember J, et al. Dual-energy computed tomography: concepts, performance, and thoracic applications. J Thorac Imaging 2012;27:7-22. [Crossref] [PubMed]
- van der Laan MJ, Bakker CJ, Blankensteijn JD, et al. Dynamic CE-MRA for endoleak classification after endovascular aneurysm repair. Eur J Vasc Endovasc Surg 2006;31:130-5. [Crossref] [PubMed]
- Jonker FH, Verhagen HJ, Mojibian H, et al. Aortic endograft sizing in trauma patients with hemodynamic instability. J Vasc Surg 2010;52:39-44. [Crossref] [PubMed]
- Janosi RA, Gorla R, Rogmann K, et al. Validation of intravascular ultrasound for measurement of aortic diameters: Comparison with multi-detector computed tomography. Minim Invasive Ther Allied Technol 2015;24:289-95. [Crossref] [PubMed]
- Di Mario C, Madretsma S, Linker D, et al. The angle of incidence of the ultrasonic beam: a critical factor for the image quality in intravascular ultrasonography. Am Heart J 1993;125:442-8. [Crossref] [PubMed]
- Sze DY, Mitchell RS, Miller DC, et al. Infolding and collapse of thoracic endoprostheses: manifestations and treatment options. J Thorac Cardiovasc Surg 2009;138:324-33. [Crossref] [PubMed]
- van Bogerijen GH, van Herwaarden JA, Conti M, et al. Importance of dynamic aortic evaluation in planning TEVAR. Ann Cardiothorac Surg 2014;3:300-6. [PubMed]
- van Prehn J, Vincken KL, Muhs BE, et al. Toward endografting of the ascending aorta: insight into dynamics using dynamic cine-CTA. J Endovasc Ther 2007;14:551-60. [Crossref] [PubMed]
- Jonker FH, Verhagen HJ, Lin PH, et al. Open surgery versus endovascular repair of ruptured thoracic aortic aneurysms. J Vasc Surg 2011;53:1210-6. [Crossref] [PubMed]
- Higashi H, Kanki A, Watanabe S, et al. Traumatic hypovolemic shock revisited: the spectrum of contrast-enhanced abdominal computed tomography findings and clinical implications for its management. Jpn J Radiol 2014;32:579-84. [Crossref] [PubMed]
- Stein E, Mueller GC, Sundaram B. Thoracic aorta (multidetector computed tomography and magnetic resonance evaluation). Radiol Clin North Am 2014;52:195-217. [Crossref] [PubMed]
- van Prehn J, van Herwaarden JA, Muhs BE, et al. Difficulties with endograft sizing in a patient with traumatic rupture of the thoracic aorta: the possible influence of hypovolemic shock. J Vasc Surg 2008;47:1333-6. [Crossref] [PubMed]


