Surgical options to treat massive sternal defect after failed Robicsek procedure
Introduction
Full median sternotomy is still the most commonly used surgical approach for exposing the heart in cardiac surgery (1). However, early sternotomy wound complications represent a major cause of morbidity in heart surgery with an incidence from 0.5% to 10% in most surgical studies (2-4). Different classifications of sternal wound complications were reported. Mainly we consider three principal categories: mechanical uninfected sternal instability/dehiscence; sternal instability/dehiscence with superficial wound sternal infection (SWSI); and deep sternal wound infection (DSWI or mediastinitis) with sternal instability/dehiscence (1,5). In particular mediastinitis with sternal dehiscence is a life threatening condition with a reported mortality between 14% to 47% (5). The loss of sternal bone and adjacent ribs due to osteomyelitis and previous surgical debridement causes large defects of the anterior chest wall that increase the risk of heart and lung damage and in particular impairs respiratory function due to pain and paradox movement of the thorax, often necessitating prolonged mechanical ventilation and increasing the post operative mortality (6). Sometimes DSWI and massive sternal loss is the result of previous attempts to fix a mechanical sternal dehiscence with or without SWSI in patients with multiple risk factor for sternal instability and infection (6,7). In cardiac surgery the most common technique to repair a sternal instability/dehiscence is the Robicsek technique (8). Generally this is a very effective procedure, but in case of its failure the subsequent repair of the anterior chest wall could become very complex due to massive bone loss (7,9). We report our experience with two different surgical approaches to treat a massive sternal loss after a failed Robicsek repair.
Case presentation
Patient 1
A 71-year-old male referred to our Unit because of post sternotomy mediastinitis with complete sternal defect. Two months before, the patient had undergone a Bentall procedure for aortic valve insufficiency secondary to infective endocarditis, complicated by postoperative bleeding, which required surgical re-exploration. Two weeks later mechanical instability of the sternum was detected and treated surgically using the Robicsek technique. Nevertheless, a deep sternal wound infection due to Pseudomonas Aeruginosa occurred. Thus during a further re-operation the cage was removed identifying a complete dehiscence of the sternum with multiple edge fractures and bone loss. VAC therapy was applied for 15 days. The antibiotic therapy was made according to the bacterial sensitivity resulting in the patient being treated with levofloxacin 500 mg twice a day intravenously for one month. When the acute infection was resolved the patient was ready to go to the operation room, considering his preoperative condition was stable.
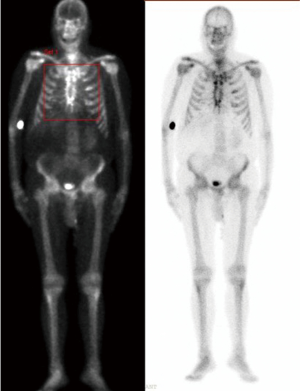
Once the VAC therapy was removed, the resection of residual edges of the sternum was performed reaching 1–2 cm into the healthy tissue. We planned before to reconstruct the anterior chest wall with a cryopreserved tibial bone allograft because of massive bone loss and the previous infection. The allograft was harvested from a multi-tissue donor, in accordance with Italian legislation for tissue donation. The graft underwent washing immersion in sterile saline solution with antibiotics for 72 hours. After packaging, the allograft was irradiated and then stored at –80 °C. The day before surgery the graft underwent defrosting at 4–6 °C for 12 hours, then under sterile conditions it was immersed in a 0.9% NaCl solution with antibiotics and stored to 4–6 °C until its use. Thus the bone was rewarmed and tailored according to the defect. To fix the allogenic bone graft and simultaneously stabilize the whole chest wall, four transversal titanium plates were used (Synthes®, Solothurn, Switzerland). Multiple holes were opened on the allograft with the drill to pass three stainless-steel wires for each titanium plate to attach the allograft to the titanium bars. Then the wire-loops were tied at an appropriate strength. A fenestrated drainage was placed under the graft before its fixation. The titanium bars were then fixed to the patient’s ribs bilaterally using titanium screws. We applied at least three screws to both ends of each plate taking care to avoid the cartilaginous part of the ribs. Once the reconstruction of the chest wall was completed, we performed bilateral release of the pectoral muscle flaps. Two Jackson-Pratt drainages were placed under both pectoral flaps, which were closed medially with interrupted sutures (Figure 1). The patient was extubated in the operation room (OR) and then transferred to the intensive care unit for monitoring. The drainages were removed after 5 days in order to avoid the formation of serous exudate. The postoperative computed tomography demonstrated perfect apposition and healing of the allograft to the recipient’s tissue. The post operative period was uneventful and the patient was discharged after 20 days from the operation in optimal medical condition. After 1 year the patient is still alive, he has no chronic pain, and the chest wall is stable with no displacement of the bars, as shown in the follow-up CT scan, which was performed 10 months after the operation, showing a stable anterior chest wall reconstruction (Figure 2). Whole-body single-photon emission computed tomography (SPECT/CT) with technetium 99m-bisphosphonate, which is an accurate assessment for the osteoblastic metabolism was performed after one year. The study confirmed no alteration of the graft, in particular necrosis; rather, initial signs of tissue healing of the bone graft in the contact points with the host bone tissue have been confirmed (Figure 3).

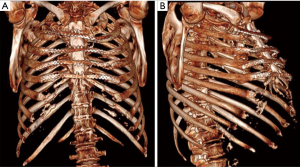
Patient 2
A 59-year-old male patient with dilated cardiomyopathy underwent to orthotopic heart transplantation at our surgical department. Reoperation for bleeding was necessary after 4 days. Two weeks later, sternal dehiscence was detected and treated surgically using the Robicsek technique. During the hospitalization, a wound infection with sternal instability was discovered, a Klebsiella Pneumoniae was isolated and the patient treated with piperacillin/tazobactam 18 mg by continuous intravenous infusion for 1 month. Following this, the patient underwent surgical re-exploration to remove the cage identifying a complete dehiscence of the sternum with multiple edge fractures and bone loss. VAC-therapy was applied for 14 days until the wound was germ-free. Once the acute phase of the infection has passed, we decided to bring the patient to the OR to reconstruct the anterior chest wall.
The operation was performed under general anesthesia. The VAC therapy was removed and the degree of sternal separation was assessed. The edges of the sternal bone were mobilized and cut until bleeding from the bone marrow was visible. Bilateral release of pectoral muscle, at least at the level of the midclavicular line, was performed for later approximation. Despite a small rim of sternal edges still being present, reconstruction using metal steel wires or titanium plate alone was not possible because of multiple fractures of the sternal bone and a very fragile and thin rim of tissue that did not guarantee any kind of stability.
To cover the anterior chest wall defect, we decided to use a titanium mesh (RPS.THORAX - MDF Medica S.r.l.). The mesh was placed above the sternal residue and fixed bilaterally to the ribs head with four steel wires. A fenestrated drainage was placed under the mesh before its fixation. To avoid paradoxical movement of the chest, three titanium plates (RPS.THORAX - MDF Medica S.r.l.) were attached to the third, fourth and fifth ribs bilaterally and to the mesh with at least three locking screws at both ends of each plate. Once the reconstruction of the chest wall was completed, the pectoralis muscles were closed with interrupted sutures. Two Jackson-Pratt drainages were placed under the pectoralis flap and two over it. The subcutaneous tissue was approximated with a running suture and the skin was then closed with staples and non-absorbable braided sutures (Figure 4).

The patient was transferred to the ICU and extubated after 8-hours. The drains were removed after 6 days in order to avoid the formation of serous exudate. There were no complications related to the operation. The postoperative computed tomography showed the device in place and the correction of the defect. The patient was discharged after 18 days in optimal medical condition.
After a 12 months follow-up examination, the patient is in good clinical condition, has no chronic pain and the chest wall is stable with no displacement of the synthetic device. The patient had a CT scan at 6 months and it showed a stable reconstruction of the chest wall (Figure 5).
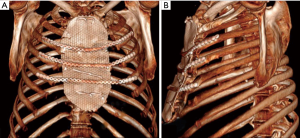
Discussion
Sternal dehiscence represents one of the major causes of morbidity after cardiac surgery performed through full median sternotomy (2-4). Sternal preservation is the better way to avoid this kind of complication (1). Particular attention should be paid to patients with well-known risk factors, such as older age, diabetes, COPD, congestive heart failure, use of bilateral internal mammary artery grafts, reoperation, prolonged ventilation, obesity, immunosuppressive therapy, severe osteoporosis, prolonged cardiopulmonary bypass and aortic cross-clamp times, etc., to prevent sternal instability (5). Then, there’s also other reasons for mechanical sternal dehiscence/instability such as an asymmetric sternotomy or repeated sternal reopening and rewiring for post-operative bleeding, or other surgical complications.
When SWSI with sternal instability/dehiscence occurs, VAC therapy followed by Robicsek closure represents the most used approach to date (2-4). The Robicsek procedure consists of creating bilateral and longitudinal parasternal running wires, with alternating sutures passed anteriorly and posteriorly to the costal cartilages for the whole length of the sternum (8). The main disadvantage is that it produces a constrictive weave which can interrupt the collateral blood supply of the sternum, worsening a pre-existing ischemia and resulting in bone necrosis and further sternal fragmentation (7). Thus, an ischemic field facilitates bacterial colonization and migration from SWSI, frequently present in the deep mediastinal space causing DSWI. Robicsek failure and DSWI requires wire removal and sternal debridement, which results in massive bone loss. Usually, this huge anterior chest wall defect is first stabilized with VAC therapy, that is also very effective in treating DSWI and SWSI, in addition to antibiotic therapy (4).
There are multiple surgical techniques described to reconstruct the anterior chest wall once the mediastinum is sterilized, but none of them is yet considered the gold-standard procedure (12,13). We described two different approaches for anterior chest wall reconstruction. In the first case, we used synthetic material such as titanium bars, screws and mesh. Synthetic materials have advantages as well as disadvantages such as excessive rigidity, the risk of erosion of the adjacent structures, the risk of infection, insufficient strength, rupture, migration, immunological reaction and impossibility of incorporation into the host tissue (12-14). Of course, synthetic materials are readily available, simple to implant and easily adaptable to the patient's anatomy. In particular, titanium is extremely biocompatible, malleable, has a low density, a high-thermal and mechanical resistance and is magnetic-resonance compatible with minimal diffraction and artifacts during computed tomography (12). In literature, there’s lots of reports describing sternal fixation using titanium bars, plates and screws (9). In our case, we used a new titanium mesh that, in patients with massive bone loss and complete absence of sternum, where titanium plates or bars would have been impossible to implant, allowed us to achieve a stable reconstruction of the anterior chest wall. This mesh has excellent biomechanical characteristics, is simple to trim for optimal adaptation, is relatively simple to fix with metal steel wire and/or titanium screws to the ribs. In very complex cases, using the preoperative CT scan images it is possible to request a custom tailored mesh in thickness and dimension/form based on a 3D printing model. In this case, we used a standard mesh because there was no particular difficulty to adapt it to the patient’s anatomy.
The second option for anterior chest wall repair, in particular in case of suspected or previously treated infection, are the biological materials. Bone autografts are optimal because of their biomechanical characteristics and compatibility but their availability is limited (14). In our previous experience and in literature, the use of bone allografts for sternum reconstruction has become more common, demonstrating good results (6,7,14-16). They also provide sufficient amount of material for reconstruction of chest wall defects of larger extent, with optimal cosmetic results. Moreover, bone allografts have the same advantage as bone autografts in terms of infection risk, compatibility, and host tissue incorporation, but they do not require additional incisions or tissue removal for harvesting. Bone grafts, theoretically also act as a scaffold for osteoprogenitor cells and bone formation but in the future, more imaging and histopathological studies will be needed to confirm osteoinduction, osteoconduction and complete osteointegration of the implanted bone allograft (17,18). The preoperative CT scan of the recipient and the allograft radiograph is used to achieve a correct match. Intraoperatively, the presence of any discrepancy between the allograft and the surgical site of implantation can be easily corrected by tailoring the bone allograft with a saw and rasp. At the end of the operation, a quick, safe and efficient stabilization of the transplanted bone is achieved with titanium plates and screws, usually placed between the allograft and the ribs. In literature, different bone allografts have been reported to have been used for sternal replacement (19,20); in this case we used a tibial segment with good result. In our experience, the kind of bone allograft used for reconstruction isn’t important because intraoperative tailoring is always possible and the biomechanical characteristics are more or less the same; mainly the decision is made by the tissue bank bone allograft availability.
In 2012, we described the first four cases of sternal allograft transplantation, three partial sternal transplantations in patients undergoing partial sternectomy for neoplastic disease and one complete sternal replacement for a massive post sternotomy defect. We had no mortality or morbidity related to the surgical technique even after a mean follow-up of 9.7 months (14).
Kalab et al. recently published (6) a series of 10 patients who underwent allogenic bone graft (sternum and head femur) for massive post sternotomy defects. In six cases, they achieved optimal results without complications, while in three patients, resuturing of the soft tissue was necessary; all patients achieved excellent chest wall stability. They reported only one death due to severe concomitant complications. At 2.3 and 18.22 months after the operation SPECT/CT with technetium 99m-bisphosphonate was performed in four patients, showing high reactivity of the graft in all patients; in one patient, a 42% reduction of the osteopenic defect of the allogenic graft was demonstrated. Also, in our first case we performed SPECT/CT with technetium 99m-bisphosphonate one year after allograft implantation and we confirmed early signs of bone integration, in particular in the contact points between the allograft and host bone.
A 2016 multicenter study, regarding 18 patients who underwent allograft sternal transplantation, reported excellent long-term results with a mean follow-up time of 36 months. None of these patients had complications related to the implanted allograft, and all showed a good chest wall stability and optimal respiratory function (16).
New technologies such as 3D-printers and computed based navigation surgery appear to be very promising in preoperative planning and simplification in matching the sternal substitute and the recipient’s chest wall (12,21).
Conclusions
Both of the presented techniques seem to be effective for reconstruction of anterior chest wall defect due to massive sternal loss after a failed Robicsek procedure. Nowadays, which material is better in the replacement of the sternum is still to be defined on the basis of studies. At the moment, the choice is based on the experience of the single surgeon and on the material available in the referral center.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Informed consent for publication was obtained previously in both patients.
References
- Douville EC, Asaph JW, Dworkin RJ, et al. Sternal preservation: A better way to treat most sternal wound complications after cardiac surgery. Ann Thorac Surg 2004;78:1659-64. [Crossref] [PubMed]
- Hosseinrezaei H, Rafiei H, Amiri M. Incidence and risk factors of sternal wound infection at site of incision after open-heart surgery. J Wound Care 2012;21:408-11. [Crossref] [PubMed]
- Cowan KN, Teague L, Sue SC, et al. Vacuum-Assisted wound closure of deep sternal infections in high-risk patients after cardiac surgery. Ann Thorac Surg 2005;80:2205-12. [Crossref] [PubMed]
- Listewnik MJ, Sielicki P, Mokrzycki K, et al. The use of Vacuum-Assisted Closure in purulent complications and difficult-to-heal wounds in cardiac surgery. Adv Clin Exp Med 2015;24:643-50. [Crossref] [PubMed]
- Schimmer C, Reents W, Berneder S, et al. Prevention of sternal dehiscence and infection in high-risk patients: A prospective randomized multicenter trial. Ann Thorac Surg 2008;86:1897-904. [Crossref] [PubMed]
- Kalab M, Karkoska J, Kaminek M, et al. Reconstruction of massive post-sternotomy defects with allogenic bone graft: four year results and experience using the method. Interact Cardiovasc Thorac Surg 2016;22:305-13. [Crossref] [PubMed]
- Dell'Amore A, Dolci G, Cassanelli N, et al. A massive post-sternotomy sternal defect treated by allograft sternal transplantation. J Card Surg 2012;27:557-9. [Crossref] [PubMed]
- Robicsek F, Daugherty HK, Cook JW. The prevention and treatment of sternum separation following open heart surgery. Coll Works Cardiopulm Dis 1977;21:61-3. [PubMed]
- Alled KB, Thourani VH, Naka Y, et al. Randomized, multicenter trial comparing sternotomy closure with rigid plate fixation to wire cerclage. J Thorac Cardiovasc Surg 2017;153:888-96.e1. [Crossref] [PubMed]
- Dell’Amore A, Campisi A, Giunta D, et al. Patient 1 allograft bone sternal reconstruction. Asvide 2018;5:582. Available online: http://www.asvide.com/article/view/25557
- Dell’Amore A, Campisi A, Giunta D, et al. Patient 2 titanium mesh sternal reconstruction. Asvide 2018;5:583. Available online: http://www.asvide.com/article/view/25558
- Sanna S, Brandolini J, Pardolesi A, et al. Materials and techniques in chest wall reconstruction: a review. J Vis Surg 2017;3:95. [Crossref] [PubMed]
- Ng CS. Recent and future developments in chest wall reconstruction. Semin Thorac Cardiovasc Surg 2015;27:234-9. [Crossref] [PubMed]
- Dell'Amore A, Cassanelli N, Dolci G, et al. An alternative technique for anterior chest wall reconstruction: the sternal allograft transplantation. Interact Cardiovasc Thorac Surg 2012;15:944-7. [Crossref] [PubMed]
- Stella F, Dell’Amore A, Dolci G, et al. Allogenic sternal transplant after sternectomy for metastasis of ovarian carcinoma. Ann Thorac Surg 2012;93:e71-2. [Crossref] [PubMed]
- Marulli G, Dell'Amore A, Calabrese F, et al. Safety and effectiveness of cadaveric allograft sternocondral replacement after sternectomy: a new tool for the reconstruction of anterior chest wall. Ann Thorac Surg 2017;103:898-905. [Crossref] [PubMed]
- Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J 2001;10 Suppl 2:S96-101. [Crossref] [PubMed]
- Granero-Molto F, Weis JA, Longobardi L, et al. Role of mesenchymal stem cells in regenerative medicine: application to bone and cartilage repair. Expert Opin Biol Ther 2008;8:255-68. [Crossref] [PubMed]
- Aranda JL, Varela G, Benito P, et al. Donor cryopreserved rib allograft for chest wall reconstruction. Interact Cardiovasc Thorac Surg 2008;7:858-60. [Crossref] [PubMed]
- Kalab M, Molitor M, Kubesova B, et al. Use of allogenous bone graft and osteosynthetic stabilization in treatment of massive post-sternotomy defects. Eur J Cardiothorac Surg 2012;41:e182-4. [Crossref] [PubMed]
- Stella F, Dolci G, Dell'Amore A, et al. Three-dimensional surgical simulation-guided navigation in thoracic surgery: a new approach to improve results in chest wall resection and reconstruction for malignant diseases. Interact Cardiovasc Thorac Surg 2014;18:7-12. [Crossref] [PubMed]


