New bullae formation in the staple line increases the risk of recurrent pneumothorax following video-assisted thoracoscopic surgery bullectomy for primary spontaneous pneumothorax
Introduction
Primary spontaneous pneumothorax (PSP) is a common condition reported in young males. Recently, video-assisted thoracoscopic surgery (VATS) has gained widespread recognition and has become the standard modality for PSP management owing to less postoperative pain, less invasiveness, shorter hospitalization, and fast return to daily routine. However, the recurrence rate of PSP remains high, and some other surgical approaches, including parietal pleurectomy, mechanical abrasion, and reinforcing the staple line with synthetic materials, have been considered (1-3).
Recently, numerous etiologies of recurrent pneumothorax following VATS have been proposed. While missed bullae have been recognized as the reason for recurrent pneumothorax following VATS, new bullae formation is now being considered an important etiology (4-7). We have previously reported that higher tension in the staple line which was associated with bulky resection during VATS, increased the risk of recurrence (7).
Typically, new bullae developed at two different sites: predominantly in the staple lines and others in the non-stapling lines. Although several previous studies have suggested that the formation of new bullae was associated with the recurrent pneumothorax following VATS, these findings did not reach a statistical significance and did not delineate the new bullae formation according to the site of bullae (1,8,9).
Thus, the aim of this study was to evaluate the etiology of recurrent pneumothorax according to new bullae formation, particularly the site of bullae.
Methods
After obtaining approval from the institutional review board, we conducted a retrospective review of the electronic medical record (EMR) system to identify patients (age, <30 years) with PSP who had undergone first-time VATS between April 1, 2009, and December 31, 2014. We identified 301 patients with 415 operated lungs. Of these, 20 patients underwent bilateral VATS for bilateral pneumothorax or unilateral pneumothorax with contralateral visible bullae identified using chest high-resolution computed tomography (HRCT) and 37 underwent contralateral VATS during the next hospitalization post first VATS. Of these 415 operated lungs, follow-up HRCT was performed in 85 cases, and they were included in this study. Of note, HRCT was reconstructed in 3-mm-thick images of the entire chest, and 1-mm-thick images of the apex were added.
We reviewed the patients’ EMRs, comprising all operative notes and pathologic reports. The findings of the chest X-ray and HRCT were reviewed using the Picture Archiving Communication System. The assessment method of the longest length and the specimen volume are described elsewhere (7). The presence of new bullae and pneumothorax in HRCT was assessed by two thoracic surgeons (C.B.P. and S.Y.C.). In addition, new bullae formation was categorized depending on the site of bullae as follows: (I) in the staple line or (II) in the non-stapling areas.
In total, 76 HRCT scans were performed for the assessment of pneumothorax symptoms and 9 scans were performed for the evaluation of other causes, such as pneumonia, pleural effusion, and so forth. Among 85 HRCT scans, ipsilateral recurrences were noted in 18 scans, contralateral recurrences were noted in 41 scans, and no pneumothorax was detected in 17 scans.
The endpoint of this study was the presence of recurrent pneumothorax in the HRCT following the first-time VATS for PSP. Patients were divided into two groups according to the presence of recurrent pneumothorax in follow-up HRCT.
Statistical analysis
Categorical variables are presented as frequencies and percentages, and continuous variables are presented as the mean ± standard deviation. Continuous variables were compared using the paired t-test and categorical variables using chi-squared or Fisher’s exact test, as applicable. In addition, the Kaplan-Meier method was used to ascertain the cumulative probabilities of recurrence along with the long-rank test of significance. Furthermore, Cox regression models were used to identify significant risk factors for recurrence. All statistical analyses were performed using the SPSS software.
Results
In this study, we examined 85 follow-up HRCT scans post the first-time VATS in PSP and 21 recurrent pneumothorax cases that were diagnosed and 60 new bullae were identified.
The mean age at the time of surgery was 18.3±3.2 (range, 14–30) years, and the majority of the patients were males (75, 88.2%). The maximum follow-up period between the index operation and HRCT follow-up was 93.5 (mean, 24.9±20.9) months.
Among 60 new bullae, 39 new bullae were located in the staple line and 38 were in other sites away from the staplers. In 17 cases, new bullae simultaneously developed in the stapling and non-stapling areas.
In the recurrence group, the mean age was significantly lower than in the non-recurrence group (17.1±1.2 vs. 18.6±3.6 years, respectively; P=0.005). However, sex, height, body weight, and body mass index exhibited no differences between the groups. In addition, the reinforcing material, such as Neoveil (Gunze Co., Ltd., Tokyo, Japan) was used at the discretion of individual surgeons, and the usage rate was not statistically different (P=0.259). The durations between the index operation and HRCT follow-up periods did not differ between the group (P=0.466) (Table 1). Furthermore, the group with new bullae in the staple lines exhibited a significantly higher recurrence rate than the group without new bullae in the staple lines (76.2% vs. 35.9%, P=0.001) (Table 1).
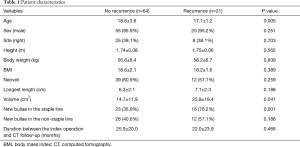
Full table
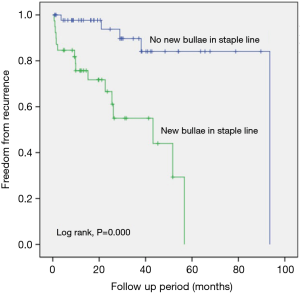
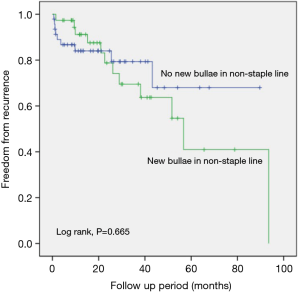
Comparison of the freedom from recurrence rates between the groups according to the presence of new bullae formation in the staple line revealed that the new bullae in the staple line group exhibited a statistically higher rate of pneumothorax recurrence following VATS than the group without new bullae in the staple line group (P=0.000, log-rank test) (Figure 1). Furthermore, new bullae formation at other sites far from the staple lines did not increase the recurrence rate of pneumothorax (P=0.665, log-rank test) (Figure 2).
We also identified independent risk factors for recurrent pneumothorax following VATS using Cox regression. New bullae in the staple line [hazard ratio (HR), 26.664; P=0.003] and resected pathology volume (HR, 1.032; P=0.020) were significant risk factors for the recurrence of pneumothorax (Table 2).
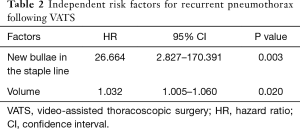
Full table
Discussion
This study primarily established that new bullae formation in the staple lines augments the risk of recurrent pneumothorax following VATS in PSP.
VATS is the standard management option for PSP because it offers several advantages, including less invasiveness, less postoperative pain, better preservation of the postoperative pulmonary function, greater cosmetic benefits, and a fast return to the routine life. Despite advancements in thoracoscopic surgical devices and staplers, the recurrence rate of pneumothorax following VATS remains high (7.0–16.3%) (1,2,4,10,11). Recently, numerous surgical procedures, such as suturing/loop ligation, parietal pleurectomy, chemical pleurodesis, mechanical abrasion, and staple line coverage using various materials, have been developed to reduce the postoperative recurrence rate (3); however, these approaches also pose a risk of recurrent pneumothorax (1.1–10.7%).
Some studies have considered the incomplete resection of oversighted blebs or bullae and lung injury during the manipulation of lungs accountable for recurrent pneumothorax following VATS (10,11). Recently, new bullae formation near the staple lines has been suggested as the key etiology of recurrent pneumothorax following VATS (1,3-7). Tsuboshima et al. (6) have reported 37.1% (26/70) rate of postoperative bulla neogenesis following VATS for PSP on the basis of bullae identification by performing HRCT at 1 year postoperatively or sooner if symptoms occurred. They have deduced that a large resected lung weight of ≥1.5 g was significantly associated with new bullae formation. Another study has attributed increased tension on the staple line during re-inflation, leading to the deformation of alveolar bronchioles near the stapling line, and the check-valve mechanism as reasons for new bullae formation around the staple line (3). Apparently, axillary minithoracotomy reduces the incidence of recurrent pneumothorax compare with VATS (12). Although the endoscopic stapler used for thoracoscopic bullectomy can result in the resection of excessive unnecessary parenchymal tissue at the opposite site, as the bullae are grasped with a single clamping, axillary minithoracotomy facilitates minimal resection with dual clamping on both margins of bullae. In addition, limited parenchymal resection by axillary minithoracotomy may lead to reduced tension on resected margin and may also be related to the lower postoperative recurrence. Previously, we have reported that a large volume of resected specimen was significantly associated with recurrent pneumothorax following VATS (7). Tsuboshima et al. and our studies (6,7) have validated the findings that a large volume of the specimen was associated with new bullae formation and with recurrent pneumothorax, respectively. However, prior studies (5-7) have not established an association between the formation of new bullae following surgery and the recurrent pneumothorax. In this study, we established an association between new bullae formation and recurrent pneumothorax following VATS for PSP.
Muramatsu et al. (1) have investigated 39 cases of recurrent pneumothorax following 449 VATSs for PSP and have reported new bullae in 37 cases; 19 cases were associated with the staple line, and 15 were not associated with the staple line, and 4 were supposedly oversighted. In addition, they observed new bullae formation within the staple line in patients with recurrent pneumothorax, indicating that the staple line reinforcement was essential to decrease recurrence rate. Reportedly, new bullae can develop at two different sites. In a study by Cho et al. (4), 26 (34%) patients showed new bullae at the stapling line and 36 (47%) showed new bullae at a location other than the staple line among a cohort of patients who underwent repeated VATS for ipsilateral recurrent PSP following VATS; these findings corroborate with those of our study. In this study, among 60 new bullae identified using HRCT, new bullae formation did not differ according to the site of bullae (Stapling: non-stapling =39:38).
The presence of tension at the stapling line has been considered as the etiology of new bullae at the staple line. However, etiology of new bullae at a site other than the staple line is similar to that of the first time spontaneous pneumothorax. Our study demonstrated that recurrence rate in patients with new bullae at the staple line was a higher than that in patients without new bulla at the staple line (Figure 1) and that the freedom from the recurrence rate in patients with new bullae at a site other than the staple line was not different to that in patients without new bullae at a location other than the stapling line (Figure 2). These findings signify that new bullae formation in the staple line was more important for the recurrence of pneumothorax than that in the non-staple line and that mechanisms underlying the development of new bullae were different for bullae at different sites.
This study has some limitations that are inherent in any such retrospective analysis. First, HRCT was not performed in all patients following VATS. Our patients were relatively young (18.3±3.2 years), and routine HRCT may have increased the probability of radiation exposure. However, almost patients with recurrent pneumothorax were enrolled in this study. Of note, the likelihood of overlooking recurrent pneumothorax was rare because most patients with symptoms were examined using HRCT. Second, the rate of new bullae formation and recurrence rate of pneumothorax in this study were overvalued than rates evaluated in the real world setting because almost cases of recurrent pneumothorax were included and because the association between new bullae formation and pneumothorax recurrence was robust.
In conclusion, new bullae formation in the staple line increases the risk of recurrent pneumothorax following VATS for PSP. Therefore, the current concept of the resection of bullae with a sufficient margin should be re-evaluated, and other procedures for decreasing the tension at the stapling line should be established.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study has obtained approval from the institutional review board (OC17RESE0137). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Muramatsu T, Ohmori K, Shimamura M, et al. Staple line reinforcement with fleece-coated fibrin glue (TachoComb) after thoracoscopic bullectomy for the treatment of spontaneous pneumothorax. Surg Today 2007;37:745-9. [Crossref] [PubMed]
- Lee S, Kim HR, Cho S, et al. Staple line coverage after bullectomy for primary spontaneous pneumothorax: a randomized trial. Ann Thorac Surg 2014;98:2005-11. [Crossref] [PubMed]
- Muramatsu T, Nishii T, Takeshita S, et al. Preventing recurrence of spontaneous pneumothorax after thoracoscopic surgery: a review of recent results. Surg Today 2010;40:696-9. [Crossref] [PubMed]
- Cho S, Jheon S, Kim DK, et al. Results of repeated video-assisted thoracic surgery for recurrent pneumothorax after primary spontaneous pneumothorax. Eur J Cardiothorac Surg 2018;53:857-61. [Crossref] [PubMed]
- Tsuboshima K, Nagata M, Wakahara T, et al. Association between postoperative bulla neogenesis at the staple line and resected lung weight for primary spontaneous pneumothorax: a retrospective study using the inverse-probability of treatment weighted method in patients grouped according to age. J Thorac Dis 2016;8:3676-81. [Crossref] [PubMed]
- Tsuboshima K, Nagata M, Wakahara T, et al. Relationship between postoperative bulla neogenesis at the staple line and the resected lung volume in primary spontaneous pneumothorax. Gen Thorac Cardiovasc Surg 2015;63:572-5. [Crossref] [PubMed]
- Choi SY, Kim Y, Kim DY, et al. Influence of lung resection volume on risk of primary spontaneous pneumothorax recurrence. J Thorac Dis 2018;10:1622-7. [Crossref] [PubMed]
- Sakamoto K, Takei H, Nishii T, et al. Staple line coverage with absorbable mesh after thoracoscopic bullectomy for spontaneous pneumothorax. Surg Endosc 2004;18:478-81. [Crossref] [PubMed]
- Cho S, Ryu KM, Jheon S, et al. Additional mechanical pleurodesis after thoracoscopic wedge resection and covering procedure for primary spontaneous pneumothorax. Surg Endosc 2009;23:986-90. [Crossref] [PubMed]
- Jeon HW, Kim YD, Kye YK, et al. Air leakage on postoperative day: powerful factor of postoperative recurrence after thoracoscopic bullectomy. J Thorac Dis 2016;8:93-7. [PubMed]
- Ohno K, Miyoshi S, Minami M, et al. Ipsilateral Recurrence Frequency after video-assisted thoracoscopic surgery for primary spontaneous pneumothorax. Jpn J Thorac Cardiovasc Surg 2000;48:757-60. [Crossref] [PubMed]
- Kim KH, Kim HK, Han JY, et al. Transaxillay minithoracotomy versus video-assisted thoracic surgery for spontaneous pneumothorax. Ann Thorac Surg 1996;61:1510-2. [Crossref] [PubMed]


