Bivalirudin versus heparin in percutaneous coronary intervention—a systematic review and meta-analysis of randomized trials stratified by adjunctive glycoprotein IIb/IIIa strategy
Introduction
Heparin has traditionally been the anticoagulant of choice for percutaneous coronary intervention (PCI). The addition of glycoprotein IIb/IIIa inhibitors (GPIs) to heparin in early trials led to a decrease in ischemic complications, but at the expense of an increase in major bleeding (1-3). Subsequently numerous studies have compared bivalirudin, a direct thrombin inhibitor, with the combination of heparin and GPIs. These trials demonstrated a reduction in major bleeding and more stent thrombosis with bivalirudin compared to heparin, but it is unclear if this is related to the confounding effect of GPI use in the heparin arm. More contemporary trials have aimed to compare bivalirudin to heparin with either no planned GPI use (4-12) or matched GPI use in both arms (8,13-16) but results have been mixed. Therefore, we performed a systematic review and meta-analysis of all randomized controlled clinical trials comparing bivalirudin and heparin, stratified by GPI use strategy.
Methods
Data sources and search strategy
We searched PubMed, EMBASE, Cochrane, CINAHL and Web-of-science databases for randomized clinical trials (RCT) published between January 2000 and December 2017 using the following search terms—“acute coronary syndrome (ACS)”, “bivalirudin”, “percutaneous coronary intervention (PCI)”, “heparin”, “glycoprotein inhibitors”, “GPI”, “mortality” and their combinations. We limited our search to English language and studies including adult population only. We also searched clinicaltrials.gov and reviewed the reference list of relevant articles. The methodology has been validated and published in previous studies (17).
Study selection
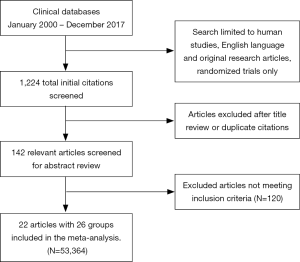
To be eligible, studies had to meet the following eligibility criteria: (I) RCT, (II) age >18 years of age, (III) compare bivalirudin to heparin with or without GPI usage (IV) report the estimate of relative risk (RR) with 95% confidence interval (CI), or other measures of RR such as hazard ratio or odds ratio or provide other forms of data from which RR could be computed. The final inclusion group consisted of 22 studies with a total of 26 comparison groups. Our search strategy is displayed in Figure S1.
Data extraction
Two reviewers (D Anugula and NR Gujjula) independently reviewed the abstracts, titles of individual studies and all selected full-length articles identified by the above-mentioned search strategy to include/exclude studies. The reviewers also independently abstracted the study characteristics, design, methods, and relevant outcomes. Any discrepancy between the first and second authors was resolved by consensus or consulting with a third reviewer (M Anantha-Narayanan).
Patient selection
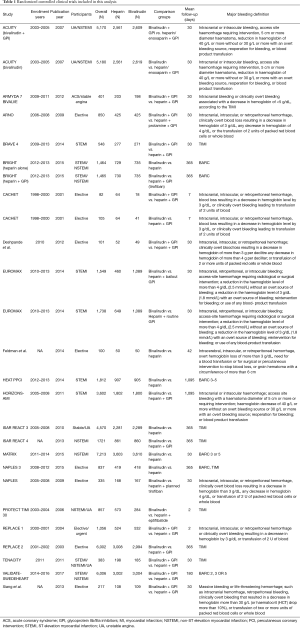
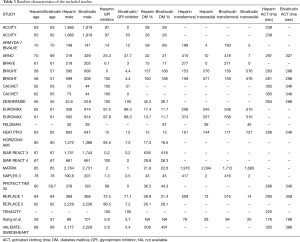
Our study included adult patients with PCI who received heparin or bivalirudin with or without GPIs as shown in Table 1 and the individual trial inclusion/exclusion criteria is shown in Table 2. We then analyzed the studies based on GPI usage. When studies had more than two comparison groups, we compared the individual to the common sub-groups. Following the overall analysis, we excluded studies that used GPIs in the heparin or bivalirudin arms and studied their outcomes to enable head-to-head comparison between bivalirudin and heparin. The usage of GPI in the individual trials is mentioned in Table 3. We performed a separate analysis of elective PCI and PCI in ACS and reported results for outcomes of interest. We then divided the studies based on radial access. We considered >60% radial access as predominant radial access and studied major bleeding outcomes in these trials.
Full table

Full table
Full table
To assess the effect of ACT in the heparin arm on overall outcomes, we performed a meta regression using the wide range of ACTs used in the trials. We then analyzed studies that reported 30-day and 1-year mortality separately. When 1-year mortality data was not available, we manually extracted these numbers from Kaplan-Meyer curve using methods similar to what was previously described (18).
Outcomes
The primary outcome was major bleeding compared between heparin and bivalirudin, with or without the use of GPI in one or both the arms. Secondary outcomes included all-cause mortality, target vessel revascularization (TVR), stent thrombosis, stroke rates and myocardial infarction at follow-up. All-cause mortality was defined as death from any cause. Major bleeding was defined in the studies as listed in Table 1.
Statistical analyses
Categorical data were pooled using the random effects model; with the pooled effect size represented as risk ratio (RR) with 95% confidence interval (CI) limits. Publication bias was assessed visually using funnel plot. Cochrane’s Q-statistics were used to determine the heterogeneity of included studies for each outcome. I2 values of <25%, 25–50%, and 50–75% were considered as low, moderate, and high heterogeneity, respectively. An exclusion-sensitivity analysis was included for heterogeneity, when necessary. A meta-regression was performed when necessary to analyze the impact of moderator variables on outcomes of interest. A P value of <0.05 was considered statistically significant. Analyses were performed by M Anantha-Narayanan using the Software Comprehensive Meta-Analysis (version 3.3) (19). This study was exempt from Institutional Review Board approval at our institution.
Results
Study characteristics
Initially, there were 23 original studies (4,5,7-16,20-30) but we excluded the BAT/HAS trial (20) as patients in this trial had received only balloon angioplasty without stents. Also, the trial did not meet our inclusion criteria for year. Finally, we had 22 original studies with a total of 26 comparison groups. Table 1 shows the baseline study characteristics and patients groups used in the analysis.
Patients
The overall study population consisted of 53,364 patients extracted from 26 comparison groups (22 original studies and 4 subgroups) and about 66% were males. Mean follow-up time was 192±303 days with maximum follow-up of 1,095 days. Mean age of the entire cohort was 63±4 years. Concomitant GPI therapy was used in about 44% of the overall patient population, 27% in the bivalirudin arm and 61% in the heparin arm. Inclusion and exclusion criteria of the individual studies are mentioned in Table 2. A total of 16 comparison groups had planned GPI usage in the heparin arm and two of the groups used only bailout GPI. Remaining studies did not have GPI in the heparin arm. A total of 17 comparison groups had bivalirudin without GPI or only with bailout GPI whereas 9 groups had bivalirudin with provisional GPI.
Major bleeding-bivalirudin versus heparin
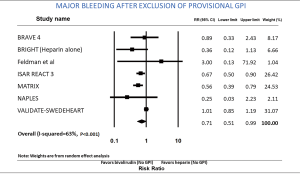
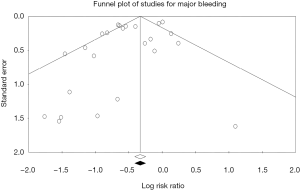
Among the overall patient population, the risk of major bleeding was 36% lower in patients receiving bivalirudin when compared to those assigned to receive heparin (RR: 0.64; 95% CI: 0.53–0.77, P<0.001) (Figure 1). After exclusion of GPI usage (both provisional and routine use) in both arms, the risk of major bleeding was still 29% lower in the bivalirudin arm than in the heparin arm (RR: 0.71; 95% CI: 0.51–0.99, P=0.041) (Figure 2). Major bleeding was still lower in the bivalirudin arm when we analyzed only studies with GPI usage (provisional and routine) in both arms (RR: 0.58; 95% CI: 0.42–0.81, P=0.001) (not shown). Sensitivity analysis with exclusion of the study (12) with the maximum strength did not alter the results (RR: 0.64; 95% CI: 0.53–0.77, P<0.001). Funnel plot showed minimal bias (Figure S2) and heterogeneity within the included studies was high (I2=65%). Analysis of studies with PCI in ACS showed lower major bleeding with bivalirudin (RR: 0.64; 95% CI: 0.53–0.78, P<0.001) but in elective PCI, the difference became insignificant between the groups (RR: 0.58; 95% CI: 0.28–1.18, P=0.130). We performed a test for interaction dividing the studies in sub groups as studies using >10% GPI (routine or provisional) in both arms and studies using <10% GPI (routine or provisional) in both arms. The overall test for interaction between the sub groups was insignificant, therefore, the statistical heterogeneity in the overall meta-analysis for major bleeding was not explained by the subgroup analyses with respect to GPI usage and there was no significant interaction.

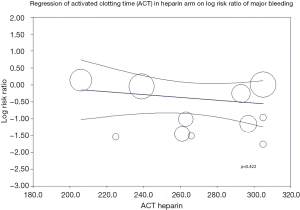
A meta-regression of ACT in heparin arm on incidence of major bleeding (Figure S3) was statistically insignificant (P=0.422) indicating different ACT levels in heparin arm does not affect the risk of major bleeding. Analysis of radial access predominant studies (studies with >60% radial access) showed a trend towards lower risk of major bleeding with bivalirudin but was statistically insignificant (RR: 0.76; 95% CI: 0.45–1.26, P=0.285).
All-cause mortality-bivalirudin versus heparin
All-cause mortality was compared from 23 studies. All-cause mortality was not different between the bivalirudin and heparin arms (RR: 0.93; 95% CI: 0.82–1.05, P=0.260) (Figure 3). Sensitivity analysis with exclusion of the study (12) with the maximum strength did not change the results of the analysis. Funnel plot showed minimal bias (not shown) and I2 was 0 (P=0.516). A meta-regression of all-cause mortality on follow-up time period was insignificant (P=0.931). When we excluded provisional and routine GPI in both arms, all-cause mortality was still similar in both groups (RR: 0.98; 95% CI: 0.84–1.15, P=0.823) (not shown).
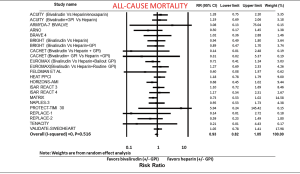
All-cause mortality was not different between the groups when we divided studies into PCI in ACS (RR: 0.95; 95% CI: 0.83–1.08, P=0.412) and elective PCI (RR: 0.73; 95% CI: 0.46–1.18, P=0.200).
We then separated studies that reported 30-day mortality and 1-year mortality as mentioned in the methods section. Both 30-day (RR: 0.94; 95% CI: 0.79–1.12, P=0.495) and 1-year (RR: 0.83; 95% CI: 0.65–1.07, P=0.160) mortality were not different between the two groups.
Stent thrombosis-bivalirudin versus heparin
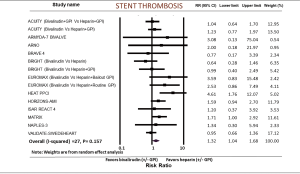
Stent thrombosis was reported in 15 studies. Risk of stent thrombosis was higher in the bivalirudin group (RR: 1.32; 95% CI: 1.04–1.68, P=0.022) (Figure 4). I2 was 27% (P=0.157). We then analyzed acute and sub-acute stent thrombosis from the available trials. Acute stent thrombosis was higher in the bivalirudin group (RR: 1.54; 95% CI: 1.07–2.23, P=0.020) whereas sub-acute stent thrombosis showed no difference between bivalirudin and heparin (RR: 1.04; 95% CI: 0.60–1.81, P=0.879). When we excluded studies with GPIs in both arms, the difference became statistically insignificant (RR: 1.40; 95% CI: 0.66–2.97, P=0.379) (not shown). In the PCI group for ACS, stent thrombosis was higher in the bivalirudin group (RR: 1.32; 95% CI: 1.02–1.73, P=0.038), whereas in the elective PCI group, there was no difference in stent thrombosis between the two groups (RR: 1.65; 95% CI: 0.51–5.35, P=0.405).
Myocardial infarction-bivalirudin versus heparin
Myocardial infarction was analyzed from 24 comparison groups comparing bivalirudin to heparin. The risk of myocardial infarction was not different between the two groups (RR: 1.12; 95% CI: 0.98–1.28, P=0.098) (Figure 5) and exclusion of GPI in both arms did not alter the difference (RR: 1.08; 95% CI: 0.90–1.30, P=0.392). Sensitivity analysis with exclusion of the study (12) with the maximum strength did not alter the results. Heterogeneity within the included studies was high (I2=60). When we divided the studies to analyze PCI in ACS, incidence of myocardial infarction was higher in the bivalirudin group (RR: 1.18; 95% CI: 1.01–1.37, P=0.041) whereas in elective PCI group, there was no difference in myocardial infarction between the two groups (RR: 0.95; 95% CI: 0.74–1.21, P=0.674).

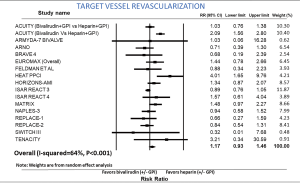
TVR-bivalirudin versus heparin
Incidence of TVR was reported in 17 studies. The risk of TVR was not different between the two groups (RR: 1.17; 95% CI: 0.93–1.46, P=0.174) (Figure 6). After exclusion of routine and provisional GPI in both arms, incidence of TVR remained similar between the two groups (RR: 1.21; 95% CI: 0.95–1.54, P=0.118). Sensitivity analysis with exclusion of the study (9) with the maximum strength did not alter the results. Heterogeneity within the included studies was high (I2=64). In PCI for ACS, there was a trend towards higher incidence of TVR in the bivalirudin group without statistical significance (RR: 1.26; 95% CI: 0.97–1.65, P=0.082). In elective PCI, there was no difference in the incidence of TVR between the bivalirudin and heparin groups (RR: 0.85; 95% CI: 0.60–1.20, P=0.357).
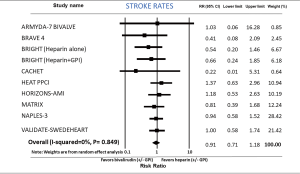
Stroke rates-bivalirudin versus heparin
Stroke rates were not different between bivalirudin and heparin group (RR: 0.91; 95% CI: 0.71–1.18, P=0.490) analyzing 10 comparison groups (Figure 7). I2 was 0 (P=0.849). After exclusion of GPI in both study arms, incidence of stroke was not different between the two groups (RR: 0.92; 95% CI: 0.69–1.21, P=0.541). Dividing the studies into elective PCI (RR: 0.94; 95% CI: 0.59–1.51, P=0.805) and PCI in ACS (RR: 0.91; 95% CI: 0.67–1.24, P=0.565) did not alter the outcomes.
Discussion
Results from our current meta-analysis provide new insight into the role of bivalirudin versus heparin in PCI. Bivalirudin use is associated with lower risk of major bleeding regardless of GPI usage in the heparin arm. This persisted even while retaining studies with GPI use in the bivalirudin arm which would have been expected to bias results towards the null. Although bivalirudin appeared to have a higher rate of stent thrombosis, analysis of studies without GPI usage in both arms showed no significant difference between the two groups. Analysis of studies only with elective PCI showed no difference in major bleeding between heparin and bivalirudin but bivalirudin showed lower bleeding in the ACS setting. Stent thrombosis was higher with bivalirudin in ACS but not in elective PCI. Bivalirudin was similar to heparin with respect to all-cause mortality, recurrent myocardial infarction, TVR or stroke rates.
Bivalirudin has been extensively studied in multiple RCTs as an acute therapy in place of heparin for patients receiving PCI. Whereas unfractionated heparin potentiates anti-thrombin III thereby inactivating thrombin and factor Xa, it has a very limited effect on clot bound thrombin. Heparin also increases platelet activation and can cause heparin induced thrombocytopenia (HIT). Comparatively, bivalirudin is a direct thrombin inhibitor acting on both clot bound and unbound thrombin (31), does not increase platelet activation and does not cause HIT.
The ACUITY (32), EUROMAX (6) and HORIZONS-AMI (27) were large RCTs that showed lower rates of major bleeding with bivalirudin when compared to heparin. However, all of these trials had GPI use in the heparin arm confounding any direct comparisons between heparin and bivalirudin. With the advancement in stent technology along with more potent P2Y12 therapy (ticagrelor and prasugrel), it is generally felt that earlier trials showing the benefit of the addition of GPI with heparin are outdated (1-3). Thus, the question of heparin monotherapy versus bivalirudin monotherapy remained unanswered.
Following these trials supporting bivalirudin, trials began to address the GPI confounding issue in the heparin arms. This resulted in a few negative RCTs. The HEAT PPCI trial (8) was designed to minimize the effect of GPI on outcomes comparing bivalirudin to heparin. In this predominantly radial trial (radial access in 80% bivalirudin and 82% heparin arms), there was no bleeding advantage to bivalirudin, and heparin was actually associated with lower thrombotic events including MI, stent thrombosis and TVR. Similarly, in the BRAVE-4 trial (25), where patients were randomized to bivalirudin plus prasugrel compared to heparin plus clopidogrel, the usage of GPI was much lower in both arms (3% heparin arm, 6.1% bivalirudin arm). The trial showed no benefit with respect to major bleeding or ischemia with bivalirudin but was confounded by the higher potency of prasugrel compared to clopidogrel between the two arms.
As the results of the above trials would suggest, it is unclear whether the bleeding benefit and increase in acute stent thrombosis with a trend towards more TVR and MI seen with bivalirudin is a real finding or as a result of confounding from GPI use in the heparin arm. In our meta-analysis, even after exclusion of trials with concomitant GPI in both arms, there was still a 48% reduction in rates of major bleeding. This increase in major bleeding with heparin did not translate to increased mortality. One could also question whether the bleeding benefit of bivalirudin would exist in the current era with a high utilization of transradial access (33). However, in the contemporary BRIGHT trial, bivalirudin showed a bleeding advantage even though the majority of the patients in the trial (79%) had radial access. Another interesting finding is that bivalirudin showed lower bleeding in the ACS trials but not in elective PCIs. The acute inflammatory state in ACS altering hemostasis increases bleeding risk where bivalirudin demonstrates benefit. In elective PCI, the absence of baseline increased bleeding risk essentially limits us from seeing a meaningful difference between the two groups even if there is truly a small lower biological bleeding risk with bivalirudin. We also sub-stratified patients by studies with radial predominant access and were unable to demonstrate a significant benefit to bivalirudin on bleeding rates in this subgroup, although the point estimate still favored bivalirudin. Whether the potential physiologically lower bleeding rates with bivalirudin are of relevance when radial access is used for PCI is therefore unclear.
Stent thrombosis rates were higher as shown in the previous meta-analysis (34) but with exclusion of concomitant GPI usage, this difference became insignificant suggesting again a confounding effect of GPI use in the heparin arm decreasing acute stent thrombosis. It is also possible that bivalirudin is intrinsically associated with a higher rate of stent thrombosis in the previously published trials that has been overcome by more potent antiplatelet therapy. In addition, other thrombotic complications including recurrent MI and TVR showed a trend towards increase with bivalirudin supporting this hypothesis. Of note, the higher thrombotic events with bivalirudin appears to be of significance not during elective PCI but only in the high risk acute ACS period where the risk-benefit ratio of anticoagulation has an even narrower therapeutic window than usual. It may be that the prothrombotic state of an MI may increase the risk of acute stent thrombosis if bivalirudin is used with its short half-life before adequate platelet inhibition has been achieved with a single dose of an oral P2Y12 inhibitor. This was seen in EUROMAX where bivalirudin was associated with higher rates of stent thrombosis in STEMI patients undergoing PCI (6). It is well known that in STEMI patients, the anticoagulation cascade gets altered and P2Y12 inhibition takes long than usual. In this trial, oral P2Y12 inhibitors were administered at approximately 50 min before PCI but complete P2Y12 inhibition occurred only at 140 min after administration. This effect along with stopping bivalirudin (which has a short half-life) early, leads to an anti-coagulation free period, increasing risk of stent thrombosis as evidenced in the HEAT-PPCI trial (8). Prolonged bivalirudin infusion regimens as used in the BRIGHT trial (4) without an increase in bleeding may be worthy of investigation as methods to reduce this potential acute stent thrombosis risk.
Although a prolonged bivalirudin infusion regimen was used in EUROMAX (6), a lower dose infusion was used which could have potentially contributed to the higher stent thrombus rates seen with bivalirudin post PCI. The higher infusion dose of 1.75 mg/kg/h was used only in one fifth of the patient population whereas the rest of the patients were given a lower dose of 0.25 mg/kg/h. This was confirmed in a post-hoc analysis of EUROMAX (6) which showed higher bivalirudin infusion dose during prolonged infusion significantly reduced the risk of stent thrombus seen with bivalirudin (35). In MATRIX, there was no difference between prolonged and shorted infusion of bivalirudin with respect to stent thrombus but only one third of the patients received a higher dose of infusion (10). If bivalirudin is used, there is currently uncertainly about the need for post procedure infusion, the dosage and duration which requires further investigation. In addition to the above-mentioned mechanisms, the altered hemostasis profile during ACS may also be contributing to bivalirudin’s lower potency causing increased stent thrombosis. Our meta-analysis suggests that this may not be applicable to elective PCI where both heparin and bivalirudin appear largely equivalent.
The recently published VALIDATE-SWEDEHEART (12), a large registry-based randomized controlled trial, showed lack of benefit with bivalirudin when compared to heparin in the absence of provisional GPI use. The trial had approximately 3,000 patients in each arm with options for pre-randomization heparin and prolonged bivalirudin infusion along with potent P2Y12 inhibitors including prasugrel, ticagrelor or intravenous cangrelor. However, this trial was also confounded by the use of heparin at a mean dose of 3,470 units in 90% of patients regardless of bivalirudin assignment, essentially comparing bivalirudin plus low dose heparin with heparin monotherapy. This would mitigate the bleeding advantage of bivalirudin monotherapy. The lack of an increase in stent thrombosis with bivalirudin in that trial is likely also influenced by this concomitant use of low dose heparin in the bivalirudin arm, but also by the use of prolonged bivalirudin infusion in two thirds of patients similar to the BRIGHT trial (36). It should be noted that even after inclusion of the trial in the meta-analysis, bivalirudin still appear to retain benefit with respect to major bleeding events but did carry a higher risk of stent thrombosis.
From the results, it may be seen that tailored anti-coagulation therapy based on individual’s risk factors may be beneficial. Advanced age, female gender, history of major bleeding in the past and renal insufficiency are associated with higher bleeding rates and so bivalirudin may be beneficial (37). Stent thrombosis has been shown to be higher in patients with ACS, long segment disease with multiple stents, small vessel diameter, bifurcation lesions and chronic total occlusions (38) and in these patients, heparin without GPIs or judicial use of bivalirudin can be options.
The strength of our meta-analysis is the inclusion of only RCTs to avoid patient selection bias. Another strength of the study is the stratification of results with and without GPIs, and also based on elective versus ACS setting. The results of our meta-analysis are not representation of the real-world registries. The variable definitions used across the studies for major bleeding create bias in comparison of multiple trials. The variable timing and dosage of P2Y12 inhibitors could affect interpretation of results. We did not have patient level data to assess outcomes for sub-groups like radial or femoral access. None of the studies were blinded. BRAVE-4 trial (25) was terminated early due to slow recruitment. Finally, publication bias is an inherent limitation of meta-analysis.
Conclusions
In summary, this systematic review and meta-analysis of published RCTs supports the use of bivalirudin in PCI. Bivalirudin is associated with lower rates of major bleeding independent of the use of GPI with no difference in other clinical outcomes when compared to heparin. The increase in stent thrombosis in earlier trials with bivalirudin is no longer apparent in the elective setting and may have been related to the inflammatory state causing increased thrombosis in ACS and also confounding in the heparin arm from GPI inhibitors lowering stent thrombosis risk. The true benefits of bivalirudin in the current era of radial PCI is an evolving question that will need further exploration.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Pertinent disclosures are included above. None of the results of this manuscript have been disclosed.
References
- Investigators E. Randomised placebo-controlled and balloon-angioplasty-controlled trial to assess safety of coronary stenting with use of platelet glycoprotein-IIb/IIIa blockade. Lancet 1998;352:87-92. [Crossref] [PubMed]
- Therapy EIESotPIIRwI. Novel dosing regimen of eptifibatide in planned coronary stent implantation (ESPRIT): a randomised, placebo-controlled trial. Lancet 2000;356:2037-44. [Crossref] [PubMed]
- De Luca G, Suryapranata H, Stone GW, et al. Abciximab as adjunctive therapy to reperfusion in acute ST-segment elevation myocardial infarction: a meta-analysis of randomized trials. JAMA 2005;293:1759-65. [Crossref] [PubMed]
- Han Y, Guo J, Zheng Y, et al. Bivalirudin vs heparin with or without tirofiban during primary percutaneous coronary intervention in acute myocardial infarction: the BRIGHT randomized clinical trial. JAMA 2015;313:1336-46. [Crossref] [PubMed]
- Lincoff AM, Kleiman NS, Kottke-Marchant K, et al. Bivalirudin with planned or provisional abciximab versus low-dose heparin and abciximab during percutaneous coronary revascularization: results of the Comparison of Abciximab Complications with Hirulog for Ischemic Events Trial (CACHET). Am Heart J 2002;143:847-53. [Crossref] [PubMed]
- Steg PG, van't Hof A, Hamm CW, et al. Bivalirudin started during emergency transport for primary PCI. N Engl J Med 2013;369:2207-17. [Crossref] [PubMed]
- Feldman A, Suleiman K, Bushari L, et al. Bivalirudin versus Unfractionated Heparin during Percutaneous Coronary Intervention in Patients at High Risk for Bleeding. Int J Angiol 2014;23:227-32. [Crossref] [PubMed]
- Shahzad A, Kemp I, Mars C, et al. Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single centre, randomised controlled trial. Lancet 2014;384:1849-58. [Crossref] [PubMed]
- Schulz S, Mehilli J, Ndrepepa G, et al. Bivalirudin vs. unfractionated heparin during percutaneous coronary interventions in patients with stable and unstable angina pectoris: 1-year results of the ISAR-REACT 3 trial. Eur Heart J 2010;31:582-7. [Crossref] [PubMed]
- Valgimigli M, Frigoli E, Leonardi S, et al. Bivalirudin or Unfractionated Heparin in Acute Coronary Syndromes. N Engl J Med 2015;373:997-1009. [Crossref] [PubMed]
- Briguori C, Visconti G, Focaccio A, et al. Novel approaches for preventing or limiting events (Naples) III trial: randomized comparison of bivalirudin versus unfractionated heparin in patients at increased risk of bleeding undergoing transfemoral elective coronary stenting. JACC Cardiovasc Interv 2015;8:414-23. [Crossref] [PubMed]
- Erlinge D, Omerovic E, Fröbert O, et al. Bivalirudin versus Heparin Monotherapy in Myocardial Infarction. N Engl J Med 2017;377:1132-42. [Crossref] [PubMed]
- Patti G, Pasceri V, D'Antonio L, et al. Comparison of safety and efficacy of bivalirudin versus unfractionated heparin in high-risk patients undergoing percutaneous coronary intervention (from the Anti-Thrombotic Strategy for Reduction of Myocardial Damage During Angioplasty-Bivalirudin vs Heparin study). Am J Cardiol 2012;110:478-84. [Crossref] [PubMed]
- Deshpande NV, Pratiti R, Admane P, et al. Safety and efficacy of bivalirudin with glycoprotein IIb/IIIa for high-risk percutaneous coronary intervention. Indian Heart J 2012;64:444-8. [Crossref] [PubMed]
- Lincoff AM, Bittl JA, Kleiman NS, et al. Comparison of bivalirudin versus heparin during percutaneous coronary intervention (the Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events [REPLACE]-1 trial). Am J Cardiol 2004;93:1092-6. [Crossref] [PubMed]
- Moliterno DJ. Investigators TSCa. A randomized two-by-two comparison of high-dose bolus tirofiban versus abciximab and unfractionated heparin versus bivalirudin during percutaneous coronary revascularization and stent placement: the tirofiban evaluation of novel dosing versus abciximab with clopidogrel and inhibition of thrombin (TENACITY) study trial. Catheter Cardiovasc Interv 2011;77:1001-9. [Crossref] [PubMed]
- Anantha Narayanan M, Mahfood Haddad T, Kalil AC, et al. Early versus late surgical intervention or medical management for infective endocarditis: a systematic review and meta-analysis. Heart 2016;102:950-7. [Crossref] [PubMed]
- Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2012;12:9. [Crossref] [PubMed]
- Borenstein M, Hedges LV, Higgins JP, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010;1:97-111. [Crossref] [PubMed]
- Bittl JA, Feit F. A randomized comparison of bivalirudin and heparin in patients undergoing coronary angioplasty for postinfarction angina. Hirulog Angioplasty Study Investigators. Am J Cardiol 1998;82:43P-9P. [Crossref] [PubMed]
- Chew DP, Lincoff AM, Gurm H, et al. Bivalirudin versus heparin and glycoprotein IIb/IIIa inhibition among patients with renal impairment undergoing percutaneous coronary intervention (a subanalysis of the REPLACE-2 trial). Am J Cardiol 2005;95:581-5. [Crossref] [PubMed]
- Gibson CM, Morrow DA, Murphy SA, et al. A randomized trial to evaluate the relative protection against post-percutaneous coronary intervention microvascular dysfunction, ischemia, and inflammation among antiplatelet and antithrombotic agents: the PROTECT-TIMI-30 trial. J Am Coll Cardiol 2006;47:2364-73. [Crossref] [PubMed]
- Parodi G, Migliorini A, Valenti R, et al. Comparison of bivalirudin and unfractionated heparin plus protamine in patients with coronary heart disease undergoing percutaneous coronary intervention (from the Antithrombotic Regimens aNd Outcome [ARNO] trial). Am J Cardiol 2010;105:1053-9. [Crossref] [PubMed]
- Schulz S, Kastrati A, Ferenc M, et al. One-year outcomes with abciximab and unfractionated heparin versus bivalirudin during percutaneous coronary interventions in patients with non-ST-segment elevation myocardial infarction: updated results from the ISAR-REACT 4 trial. EuroIntervention 2013;9:430-6. [Crossref] [PubMed]
- Schulz S, Richardt G, Laugwitz KL, et al. Prasugrel plus bivalirudin vs. clopidogrel plus heparin in patients with ST-segment elevation myocardial infarction. Eur Heart J 2014;35:2285-94. [Crossref] [PubMed]
- Stone GW, White HD, Ohman EM, et al. Bivalirudin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a subgroup analysis from the Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial. Lancet 2007;369:907-19. [Crossref] [PubMed]
- Stone GW, Witzenbichler B, Guagliumi G, et al. Heparin plus a glycoprotein IIb/IIIa inhibitor versus bivalirudin monotherapy and paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction (HORIZONS-AMI): final 3-year results from a multicentre, randomised controlled trial. Lancet 2011;377:2193-204. [Crossref] [PubMed]
- Tavano D, Visconti G, D'Andrea D, et al. Comparison of bivalirudin monotherapy versus unfractionated heparin plus tirofiban in patients with diabetes mellitus undergoing elective percutaneous coronary intervention. Am J Cardiol 2009;104:1222-8. [Crossref] [PubMed]
- Xiang DC, Gu XL, Song YM, et al. Evaluation on the efficacy and safety of domestic bivalirudin during percutaneous coronary intervention. Chin Med J (Engl) 2013;126:3064-8. [PubMed]
- Zeymer U, van 't Hof A, Adgey J, et al. Bivalirudin is superior to heparins alone with bailout GP IIb/IIIa inhibitors in patients with ST-segment elevation myocardial infarction transported emergently for primary percutaneous coronary intervention: a pre-specified analysis from the EUROMAX trial. Eur Heart J 2014;35:2460-7. [Crossref] [PubMed]
- Gladwell TD. Bivalirudin: a direct thrombin inhibitor. Clin Ther 2002;24:38-58. [Crossref] [PubMed]
- Stone GW, Bertrand ME, Moses JW, et al. Routine upstream initiation vs deferred selective use of glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: the ACUITY Timing trial. JAMA 2007;297:591-602. [Crossref] [PubMed]
- Romagnoli E, Biondi-Zoccai G, Sciahbasi A, et al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: the RIFLE-STEACS (Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome) study. J Am Coll Cardiol 2012;60:2481-9. [Crossref] [PubMed]
- Cavender MA, Sabatine MS. Bivalirudin versus heparin in patients planned for percutaneous coronary intervention: a meta-analysis of randomised controlled trials. Lancet 2014;384:599-606. [Crossref] [PubMed]
- Clemmensen P, Wiberg S, Van't Hof A, et al. Acute stent thrombosis after primary percutaneous coronary intervention: insights from the EUROMAX trial (European Ambulance Acute Coronary Syndrome Angiography). JACC Cardiovasc Interv 2015;8:214-20. [Crossref] [PubMed]
- Webster MWI, Stables RH. Bivalirudin versus Heparin Monotherapy in Myocardial Infarction. N Engl J Med 2018;378:299. [PubMed]
- Moscucci M, Fox KA, Cannon CP, et al. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J 2003;24:1815-23. [Crossref] [PubMed]
- Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005;293:2126-30. [Crossref] [PubMed]


