Continuous 389 cases of Da Vinci robot-assisted thoracoscopic lobectomy in treatment of non-small cell lung cancer: experience in Shanghai Chest Hospital
Introduction
Following the widespread use of robotic-assisted surgical technology in urology, obstetrics and gynecology, and cardiac surgery, it has been widely used in thoracic tumor (1-4). Before the appearance of thoracoscope minimally invasive surgery, thoracotomy was the main approach that requires distraction of ribs (5-8). Compared with traditional open surgery, video-assisted thoracic surgery (VATS) is less invasive as it avoids damage to the structure of chest wall and distraction of ribs. VATS has less postoperative pain, shorter postoperative drainage and the hospital stay was shorter (5-12). Robotic surgery offers better maneuverability, accuracy, and stability over VATS, and provides high-definition, three-dimensional images for the surgeon. The innovative internal rotation wrist system and freely movable microsurgery enable microscopic surgical instruments to completely reproduce the human hand movements so as to achieve the coordination of hands and eyes. The system design can eliminate the adverse effect of surgeon’s hand trembling on surgery. Its greatest innovation is to make remote operation possible. Robotic surgical system has been used in thoracic surgery such as mediastinal tumor resection, esophageal tumor resection, and lung tumor resection.
This article retrospectively analyzed 389 patients receiving robotic-assisted thoracoscopic lobectomy from May 2013 to December 2016 in Shanghai Chest Hospital. The operation time, intraoperative blood loss, postoperative drainage within 3 days, postoperative extubation days, postoperative hospital stay, total cost of operation were analyzed. This study was approved by the ethics board of Shanghai Chest Hospital [KS(P)1811].
Methods
Da Vinci surgical system
Da Vinci surgical system is an advanced robotic platform that is engineered to perform complex surgeries with minimal invasiveness. Da Vinci surgical system consists of three parts: surgeon’s console, bedside robotic arm system and the imaging system. The surgeon sits in the console outside the sterile area of the operating room, and uses both hands (by operating two main controls) and feet (via foot pedal) to control the instrument and a three-dimensional high-definition endoscope. The bedside arm system is the operating part of surgical robot with primary function in providing support for the mechanical arm and camera arm. The assistant doctor works beside the bedside arm system in the sterile area and is responsible for changing the instrument and the endoscope to assist the surgeon in completing the operation. In order to ensure patient safety, the assistant doctor has higher priority over the motion of the bedside arm system than the chief surgeon. The imaging system incorporates with core processor and image processing instrument of the surgical robot, and is located outside the sterile area during the operation. It can be operated by circulating nurse and can be used for placing various types of assistive surgery devices. The surgical robotic endoscope has high-resolution three-dimensional (3D) lens, with more than 10 times of magnification in surgical field. It provides three-dimensional high-definition image in the body cavity of the patient for the surgeon, so that the surgeon can better handle the operating distance than conventional thoracoscopic surgery, with more recognition of anatomical structure and improved accuracy.
Patient data
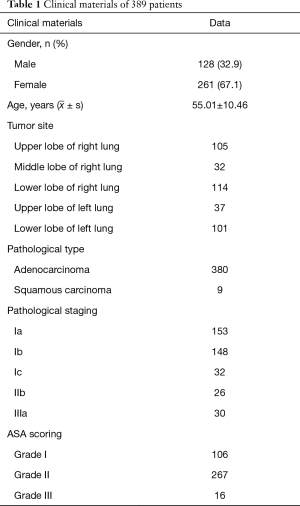
There were 261 (67.1%) females and 128 males (32.9%); aged from 20–76 years, with a mean age of 55.01 years; with ASA I in 106 cases, ASA II in 267 cases and ASA III in 16 cases; with BMI from 16.87–34.05, averaged at 23.09±2.79. The diameter in chest CT was 0.3–3.0 cm, averaged at 1.29±0.59 cm; with stage Ia in 153 cases, stage Ib in 148 cases, stage Ic in 32 cases, stage IIb in 26 cases and stage IIIa in 30 cases; with left upper lobe in 37 cases, left lower lobe in 101 cases, right upper lobe in 105 cases, right middle lobe in 32 cases and right lower lobe in 114 cases; including 380 cases of adenocarcinoma and 9 cases of squamous carcinoma (Table 1). Preoperative examinations showed no external invasion, metastasis and tolerable cardiopulmonary function. The surgical approach was decided according to the surgeon’s judgement and the patient’s own economic condition. All the 389 patients completed the surgery successfully with no conversion.
Full table
Anesthesia, posture and incision option
All the patients in this group were treated with double-lumen endotracheal intubation, general anesthesia, intraoperative single-lung ventilation and contralateral decubitus position, with patient's upper extremities in flexion and holding pillow. The operating bed was adjusted to turn the torso into slight upward-folding position to widen the intercostal space passively. Da Vinci surgery completes lobectomy and systemic lymph node dissection through the arms and auxiliary port. Position of ports: the camera port was generally in the axillary midline at 7th intercostal space. The left and right arms should be located in the same horizontal plane as the camera port, and the distance between the arms should be around 8 to 10 cm to facilitate overall motion and to reduce the direct collision of arms that would interfere with smooth surgery. The auxiliary port is preferred in the 3th or 4th intercostal space at anterior axillary line.
Surgical procedures
The camera is inserted through trocar at targeted position. After examining the thoracic cavity for no extensive adhesion, carbon dioxide is inflated to ensure clear vision and to accelerate residual gas discharging in lungs. Then two arms are placed, and the bedside arm system is docked. Generally, the right arm carries cautery hook, and the left arm carries Cadiere forceps. For the lower lobe resection with well-developed lobar fissure, the assistant lifted upper lobe vertically, then exposing artery and dissecting the 11th lymph node station. After handling the lobar fissure, the next step is to pull the lobe anteriorly to expose postmediastinum, then dissecting the 7th lymph node station and separating the lower lobar bronchi; and then the lower lobe is pulled vertically to expose and free anterior pulmonary hilus. The lower lobe is then pulled in a cephalad direction to expose and to manage the lower pulmonary ligament. The 2nd and 4th lymph node stations are dissected at the end of operation (5th and 6th stations in the left) (1).
Results
The mean operation time (from skin incise on and installing to the end of sternal closure) for robotic lobectomy was 91.51±30.80 min, ranging between 46–300 min, with estimated intraoperative blood loss of 0–100 mL in 371 cases (95.80%), 101–400 mL in 12 cases (3.60%) and >400 mL in 2 cases (0.60%); there were 4 conversions (1.2%) in which 2 cases had massive hemorrhage due to pulmonary artery branches and 2 cases had difficulty in separating due to extensive dense adhesions; there was no mortality during surgery or within 30 days after surgery.
On the first after surgery, the mean drainage was 231.39±141.87 mL; the drainage duration ranged between 2–12 d, and no patient left the hospital with chest tube; the postoperative hospital stay was 2–12 days, averaged at 4.96±1.51 days, with postoperative hospital stay >7 days in 12 cases (3.60%). The postoperative air leakage (35 cases, 9%) was the main reason for prolonged hospitalization, and there was no re-admitted case within 30 days.
All the patients underwent lymph node sampling or lymph node dissection, with lymph nodes taken in 2–9 sets, averaged at 5.69±1.46 sets, and the number of lymph nodes taken in 3–21, averaged at 9.80±3.43.
The total cost of hospitalization (including self-paying and health-care coverage) was 60,389.66–134,401.65 CNY, averaged at 93,809.23±13,371.26 CNY.
Discussion
Several studies on robotic lobectomy for lung cancer occurred from 2002 to 2010 (9-19). Melfi et al. (9) reported 107 cases of robot-assisted lobectomy, which performed systematic lymph node dissection. Previous literature showed that the results of robotic surgery were satisfactory both in terms of the incidence of complications and in terms of various statistical indicators in intraoperative and perioperative periods. The feasibility and safety of this new technique was demonstrated early in the 34 cases of robot-assisted lobectomy reported by Giulianotti et al. (10) in 2003, and the 38 cases of robot-assisted lobectomy reported by Park et al. (11) in 2006. Gharagozloo et al. (12) reported 100 cases of robot-common thoracoscopic hybrid lung cancer surgery, in which the robot-common thoracoscopic hybrid surgery was conducted in two steps. The robot was used in freeing blood vessels and pulmonary hilus and mediastinal lymph node dissection, the remaining part was excised by common thoracoscopy to complete lobectomy. In this report, the incidence of postoperative complications was as high as 21%, and 3 cases died in perioperative period. They analyzed that the reason may be there were a large number of high-risk patients. The mortality of the last 80 cases was significantly reduced, so the first 20 cases can be considered as the learning stage. They thought that robots had obvious advantages in dissecting mediastinal lymph nodes, pulmonary hilus and pulmonary vessels. Veronesi et al. (13) first reported comparative study on chest-opening (MUSCLE-SPARING incision) lobectomy with four-armed robotic lobectomy. The postoperative hospital stay in the robot group was shorter, but the operation time was longer than the chest-opening group. However, with the end of the learning period, the operation time was significantly shortened. The experience in our center showed that Da Vinci robot-assisted lobectomy offered advantages over conventional thoracoscopic surgery, mainly in 3D field of view and the unique internal wrist rotation system, which provided surgeon with more comfortable and smoother operating experience; meanwhile, the sub-damage to the surrounding tissue was less, the trauma was smaller, and recovery was faster. The patients may have less postoperative pain after robotic surgery, but this still required further prospective experiment for confirmation.
Whitson et al. (20) systematically reviewed and compared the short-term incidence of complications and long-term survival rate of thoracoscopic lobectomy and chest-opening lobectomy in treatment of early-stage NSCLC. Thoracoscopic lobectomy was thought to provide patients with significant survival benefits. The article also showed that minimally invasive surgery had less immunosuppression in patients, while the immunosuppression caused by thoracotomy may stimulate tumor growth. Only Park et al. (11) had so far reported the long-term survival of robotic lobectomy. The study followed up 325 patients undergoing robotic lobectomy in treatment of early NSCLC from 2002 and 2010, with 76% of stage I lung cancer, 18% of stage II and 6% of stage III. The median follow-up period was 27 months, and the 5-year survival rate was 80%. These limited follow-up data indicated that the survival rate of robotic lobectomy was acceptable. The 389 cases of robot-assisted thoracoscopic (RATS) lobectomy performed at our center had no recurrence in follow-up so far, which may be due to shorter follow-up period, but long-term data was still required.
There are several different approaches to robotic lung resection that have been reported so far. Park et al. used thoracoscopic technique in robotic surgery, including perforating location and anterior-to-posterior hilar approach through two thoracoscopic holes with three mechanical arms and a 4 cm-long auxiliary incision for assistance. There are also some reports on the use of hybrid “4-hole method”, that is technical means with three mechanical arm holes and an auxiliary hole. Dilewsky and Cerfolio reported on the “full-hole” robotic lung resection technique using four mechanical arms. In order to maintain the intra-thoracic pressure of carbon dioxide, only one incision was made to remove specimens at the end of the procedure. Qingquan Luo Surgery Group in Lung Tumor Clinical Medical Center in Shanghai Chest Hospital began exploring robotic lung resection surgery from 2009, starting with full-hole and the use of ultrasonic knife for free operation. The biggest disadvantage of the surgical procedure was that suction apparatus could not be used to suck the exposure. Once the suction apparatus was in, all the pulmonary lobes would be opened due to negative pressure, leaving no surgical space. Therefore, during the operation procedure, the free operation should be carefully operated. Once bleeding occurred, the operation field of vision would be very unclear, needing the stuffing with gauze to stop bleeding by compression, affecting the operation flow and extending the operation time. Another disadvantage was that many elderly patients could not tolerate “artificial pneumothorax”. The injected carbon dioxide to maintain certain pressure would affect the patient’s hemodynamics, reducing blood pressure and slowing down heart rate. Based on above preliminary exploration, we changed the surgical technique to a hybrid “4-hole method” and changed the operating instrument to electrical hook. Since the auxiliary holes can extend into common surgical instruments such as oval pliers and suction apparatus to pull lung lobes and expose field of vision, greatly simplifying the operation procedure. The fastest surgery time of lobectomy was 7 minutes. Currently, the average surgery time of lobectomy was about 45 minutes. Plus, lymph node dissection, the surgical process was controlled in 60 minutes. There has been no difference with conventional thoracoscopic surgery in surgery time.
Currently, the complete degree of lymph node dissection is a predictive factor of local recurrence. Veronesi et al. (13) and Cerfolio et al. (21) reported of no statistical difference in the number and set of lymph node dissection between robotic surgery and thoracotomy. The local recurrence rate was similar to that of thoracotomy, without significant difference. The local recurrence control was the same as the thoracotomy. These two studies also compared the thoracoscopic surgery and robotic surgery in the extent of lymph node dissection, finding no significant difference. There was no significant difference in the control of local recurrence rate. The experience in our center showed that Da Vinci robotic surgery system could perform en bloc resection of lymph nodes and their surrounding fat due to the rotating electrocoagulation hook. With clear field of view, inner rotating wrist system and operating system that filtered hand trembling, its dissection degree was higher than traditional thoracotomy. However, current comparisons on robotic, endoscopic and open surgeries were all retrospective studies. The thoroughness and safety of robotic surgical system and long-term prognosis of patients still needed prospective randomized and controlled clinical trials for confirmation.
In the 389 cases of lobectomy, we completed two cases of bronchial sleeve resection and one case of pulmonary bronchial double-sleeve resection. The flexible arm of the robot made the entire anastomosis process very smooth, and the average time for bronchial anastomosis was 15 minutes. Our advice and next step is to take full advantage of Da Vinci’s meticulous operational advantages to expand the minimally invasive thoracic surgery to previously not involved lung cancer treatment area, such as sleeve resection and angioplasty, to demonstrate its irreplaceable value factors.
There is evidence showing that robotic surgery has shorter learning curve than laparoscopic surgery. Chang et al. (22) reported that after 8–10 hours of robotic surgery training, robot operation was basically achievable. After 14 hours of training, the operating time was significantly shortened. Hernandez et al. (23) divided the surgeons into two groups according to laparoscopic surgery experience, asked them to use robot for small intestine dissection and found that there was significant difference between first and fifth small intestine dissection time. The fifth operation time was significantly shortened. Surgeons were soon proficient in robotic surgery system, which was unrelated with the surgeons’ previous laparoscopic surgery experience.
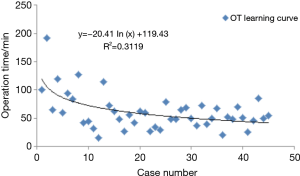
Melfi and Mussi (17) did not provide a learning curve in their report of 107 cases of robotic lobectomy, but they suggested that a minimum of 20 cases of surgical experience was needed for surgeons and surgical nurses to be adept. They also highlighted the need to standardize various steps. Based on the length of hospital stay for surgery, Gharagozloo et al. (12) also suggested that 20 surgeries were needed to obtain adequate surgical skills. Veronesi et al. (13) reported 91 cases of robotic lobectomy, in which the median surgery time and postoperative hospital stay for the first 18 patients were longer, with statistical significance, but the incidence of complications was not significantly different. The experience in our center also showed that the first 20 cases were in the learning stage, and the surgery time for the latter 20 cases was significantly shorter (Figure 1). The learning curve showed that during the initial exploring phase, the surgery time was about 120 minutes. With the accumulation of experience after about 20 surgeries, the surgeon can basically master the robotic surgery system. At present, there is no significant difference between robotic lobectomy and conventional thoracoscopic surgery in surgery time.
The study reported by Jane et al. (24) in 2011 retrospectively compared the advantages and disadvantages of robotic lobectomy and general thoracoscopic lobectomy. Their results showed that the intraoperative blood loss in the robot group was less (219 vs. 374 mL, P=0.017), and the median hospital stay was shorter (6 vs. 9, P<0.001). Their data showed that the learning curve of robotic lobectomy was shorter than that of general thoracoscopic lobectomy.
At present, the biggest problem in robotic surgery is its high surgical costs, which include the cost of robotic surgery system and the cost of disposable consumables, and this has greatly hampered the development of robotic surgery in China. In 2008, Park and Flores (25) reported that Da Vinci robotic surgery system needed one million dollars, annual maintenance cost was 100,000 dollars, and each operation was short of 730 dollars. They generally estimated that it was about 3,981 dollars more expensive than conventional thoracoscopic surgery. In developing countries like China, there are very few patients who can afford such high costs of surgery. However, as people’s income increases, the coverage of medical insurance increases, and the reimbursement ratio increases, the costs of surgery can already be accepted by most patients. At present, our center is also trying to reduce the use of disposable consumables and make full use of the robot’s unique advantages in operation, which can also greatly reduce the operation costs. China has begun to independently develop medical surgical robotic system, which I believe will challenge the current market price of robotic surgery system in the near future.
Most of the existing literature shows that robotic surgical system is safe and feasible for thoracic surgery, and the perioperative effect is similar to that of traditional video-assisted thoracoscopic surgery. However, as current development time of the surgery is still short with limited experience, the device and usage costs, necessary training of surgeons and operating room personnel, device setting time, and limited mechanical arm devices are all issues that need to be addressed. And current robot system lacks of fine force feedback, and lacks of information on mid-and-long-term prognosis. Nonetheless, more prospective randomized and controlled trials are expected in the future to prove that Da Vinci robot surgical system can improve surgical complications, pain, hospital stay and operation time, and also achieve the same mid- and long-term effects as other surgical procedures.
Acknowledgements
Funding: This work was supported by Shanghai Hospital Development Center (Grant Number: SHDC12016113).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the ethics board of Shanghai Chest Hospital [KS(P)1811].
References
- Thomas P, Doddoli C, Yena S, et al. VATS is an adequate oncological operation for stage I non-small cell lung cancer. Eur J Cardiothorac Surg 2002;21:1094-9. [Crossref] [PubMed]
- Roviaro GC, Varoli F, Vergani C, et al. State of the art in thoracospic surgery: a personal experience of 2000 video thoracoscopic procedures and an overview of the literature. Surg Endosc 2002;16:881-92. [Crossref] [PubMed]
- Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362-5. [Crossref] [PubMed]
- Förster R, Storck M, Schafer JR, et al. Thoracoscopy versus thoracotomy: a prospective comparison of trauma and quality of life. Langenbecks Arch Surg 2002;387:32-6. [Crossref] [PubMed]
- Dieter RA Jr, Kuzycz GB. Complications and contraindications of thoracoscopy. Int Surg 1997;82:232-9. [PubMed]
- Onnasch JF, Schneider F, Falk V, et al. Five years of less invasive mitral valve surgery: from experimental to routine approach. Heart Surg Forum 2002;5:132-5. [PubMed]
- Nifong LW, Chu VF, Bailey BM, et al. Robotic mitral valve repair: experience with the da Vinci system. Ann Thorac Surg 2003;75:438-42. [Crossref] [PubMed]
- Tewari A, Peabody J, Sarle R, et al. Technique of da Vinci robot-assisted anatomic radical prostatectomy. Urology 2002;60:569-72. [Crossref] [PubMed]
- Melfi FM, Ambrogi MC, Lucchi M, et al. Video robotic lobectomy. Multimedia Man Cardiothorac Surg 2005;2005:mmcts.2004.000448.
- Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery:personal experience in a large community hospital. Arch Surg 2003;138:777-84. [Crossref] [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Gharagozloo F, Margolis M, Tempesta B, et al. Robot-assisted lobectomy for early-stage lung cancer: report of 100 consecutive cases. Ann Thorac Surg 2009;88:380-4. [Crossref] [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Augustin F, Bodner J, Wykypiel H, et al. Initial experience with robotic lung lobectomy: report of two different approaches. Surg Endosc 2011;25:108-13. [Crossref] [PubMed]
- Bodner J, Wykypiel H, Wetscher G, et al. First experiences with the da Vinci operating robot in thoracic surgery. Eur J Cardiothorac Surg 2004;25:844-51. [Crossref] [PubMed]
- Zhao X, Qian L, Lin H, et al. Robot-assisted lobectomy for nonsmall cell lung cancer in China: initial experience and techniques. J Thorac Dis 2010;2:26-8. [PubMed]
- Melfi FM, Mussi A. Robotically assisted lobectomy: learning curve andcomplications. Thorac Surg Clin 2008;18:289-95. vi-vii. [Crossref] [PubMed]
- Anderson CA, Hellan M, Falebella A, et al. Robotic-assisted lung resection for malignant disease. Innovations (Phila) 2007;2:254-8. [Crossref] [PubMed]
- Giulianotti PC, Buchs NC, Caravaglios G, et al. Robot-assisted lung resection: outcomes and technical details. Interact Cardiovasc Thorac Surg 2010;11:388-92. [Crossref] [PubMed]
- Whitson BA, D’Cunha J, Andre RS, et al. Thoracoscopic versus thoracotomy approaches to lobectomy: differential impairment of cellular immunity. Ann Thorac Surg 2008;86:1735-44. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, Minnich DJ. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Chang L, Satava RM, Pellegrini CA, et al. Robotic surgery: identifing the learning curve through Objective mearsurement of skill. Surg Endosc 2003;17:1744-8. [Crossref] [PubMed]
- Hernandez JD, Bann S, Munz K, et al. Qualitative and quantitative analysis of the learning curve of a simulated task on the da Vinci system. Surg Endosc 2004;18:372-8. [Crossref] [PubMed]
- Jang HJ, Lee HS, Park SY, et al. Comparison of the early robot-assisted lobectomy experience to VATS lobectomy for lung cancer. Innovations (Phila) 2011;6:305-10. [Crossref] [PubMed]
- Park BJ, Flores RM. Cost comparison of robotic, vats and thoracotomy approaches to pulmonary. Lobectomy Thorac Surg Clin 2008;18:297-300. [Crossref] [PubMed]