Tubeless major pulmonary resections
Introduction
Following the turn of the century, modern thoracic surgery has achieved major improvements in morbidity and patient recovery through innovation aimed to reduce invasiveness. From the surgical standpoint, video-assisted thoracic surgery (VATS) has been the foremost contribution to this paradigm shift and has not ever ceased to evolve from multiportal minor resections to complex uniportal lung sparing procedures (1,2). In the meanwhile, less invasive anaesthetic techniques remained uncharted until recently.
Non-intubated spontaneous breathing under loco-regional anaesthesia has been proposed by Asian and European groups (3-8) as an alternative to double lumen lung isolation and general anaesthesia to promote faster recovery and avoid mechanical ventilation adverse effects. Although initially not considered suitable for major lung resections, lobectomies have been reported even with minimal sedation (9) and the trend has been to combine the least invasive surgical approach available with the least aggressive anaesthetic technique so far. Thus, uniportal approach is widely used alongside non-intubated anaesthesia in increasingly complex anatomic resections since first reported in 2014 (10,11).
Furthermore, a broader ‘tubeless’ concept of minimally invasive surgery and anaesthesia has been formulated (7,12). These procedures avoid the use of tracheal tube, central line, epidural or urinary catheter to improve patient comfort and allow for easier outpatient surgery. In selected patients, especially for minor resections, the chest tube can also be avoided.
Although research is gaining momentum, non-intubated spontaneous breathing resections still lack strong evidence, precise indications and technique standardization to become mainstream (13). A 2016 review and meta-analysis (14) showed that non-intubated VATS (NIVATS) was feasible and safe with a tendency to reduce length of stay and morbidity rate in patients at increased risk for general anaesthesia. Larger randomized comparative trials that include major lung resections are needed to better understand the role of NIVATS in daily practice.
Advantages and drawbacks
Proposed NIVATS benefits go beyond avoiding intubation-related trauma, mechanical ventilation induced lung injury and residual muscular blockade side- effects such as nausea and vomiting. In spontaneous ventilation, diaphragm movement provides negative pressure that improves venous return and haemodynamic stability. Dependent lung is better perfused, less prone to atelectasis and recruitment is facilitated. Ventilation perfusion mismatch and intrapulmonary shunt are reduced; hence, risk of hypoxaemia is lower. Moreover, attenuated stress response has been described (15) and NIVATS might improve antitumoral immune performance by preserving function of natural killer cells (16).
Postoperative advantages have also been found such as shorter fasting time, chest tube duration and length of stay (5,17) while tubeless perioperative protocol claims the potential to reduce patient perturbation and hospital stay even more (7,12,18,19). It is important to notice that non-intubated procedures provide a chance for surgical treatment to patients that otherwise would be rejected for intubated general anaesthesia such as emphysematous ones in the need for lung volume reduction surgery (20-22).
In the technical aspect, NIVATS can raise some concerns. While lung collapse can be as good as in double-lumen intubation, spontaneous breathing could make these procedures much technically demanding if challenges are not safely addressed by the whole team. Mediastinal shift towards dependent lung, diaphragm displacement and cough reflex triggering must be controlled before performing anatomic resections through fine adjustment of sedation and intraoperative vagal block. There is a higher risk of hypercapnia due to sedation-induced lower central responsiveness to carbon dioxide added to the re-breathing of exhaled air phenomenon. Low to moderate permissive hypercapnia is usually well tolerated with levels up to 70 mmHg (6).
Unit requirements and patient selection
Experienced, high-volume, multi-disciplinary thoracic surgery units are the best setting to introduce NIVATS practice, starting with minor procedures in the most convenient cases such as young and healthy patients with primary pneumothorax or hyperhidrosis. In advance, all the team must understand the crisis management protocol and go through sustained training (23). Surgeons must be proficient in uniportal anatomic resections and anaesthesiologists must develop lateral decubitus intubation skills. Nursing team should be involved and well aware of the procedures and their exact role on each scenario (24).
Patient selection for NIVATS has never been well stablished but has evolved from severe emphysematous patients towards a less constraining population as more complex and diverse procedures have been reported feasible (25-27). Although published algorithms have small variations in BMI and ASA thresholds, there is a steady pattern of low comorbidity, non-obese, early stage lung cancer with easy airway and lack of extensive pleural adhesions among proposals as the ideal patients (Table 1).

Full table
Anaesthetic considerations
Standard monitoring include peripheral venous access, electrocardiogram, non-invasive blood pressure, pulse oximetry, respiratory rate and end-tidal carbon dioxide estimation with nasal cannula (6). More invasive radial artery line for a more accurate blood pressure and carbon dioxide monitoring has also been reported (24). In a tubeless approach, central line and urinary catheter should also be avoided, particularly if the procedure is expected to be short. Bispectral Index (BIS) is used to target depth of sedation.
Premedication can consist of midazolam (0.15–0.25 mg/Kg) and atropine (0,01 mg/Kg) 15 min prior to anaesthesia. Proper level of sedation is achieved adjusting propofol and remifentanil infusion to keep BIS between 40 and 60. Alternatively, use of only propofol (2–4 mg/kg/h) with an effective intercostal block is also feasible, reserving fentanyl (50–100 µg) to control respiratory rate (24). Dexmedetomidine is a promising drug for NIVATS due to its profile of less respiratory depression (28).
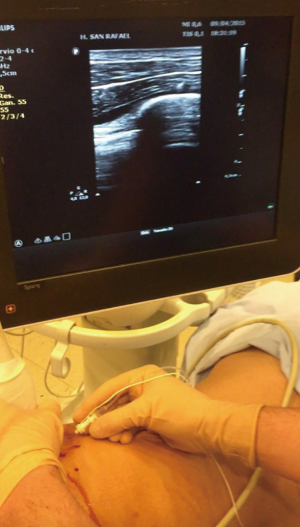
Either epidural, paravertebral or intercostal block can be used for analgesia in non-intubated procedures as long as are combined with sedation. Intercostal block has been reported to offer a significant reduction of induction time and less need of vasoactive drugs (29). A mixture of lidocaine 1% and levobupivacaine 0.2% is used to block the level of the incision and can be extended two or three intercostal spaces above and below. This procedure should be done with thoracoscopic view and can be repeated at the end of the operation in longer procedures (Figure 1). However, our preferred method to perform tubeless NIVATS is the use of paravertebral block under ultrasound guidance (Figure 2).

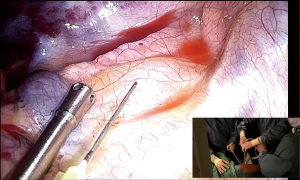
Routine blockade of vagal nerve must be done to minimize cough triggering with bronchial traction (levobupivacaine 2 mL, 0.25%). On the right side it should be performed in the paratracheal space (Figures 3,4) and in the left side at the level of the aortopulmonary window (Figure 5). It is mandatory that anatomical resections are made under full cough abolition to safely dissect hilar structures.

Supplementary oxygenation can be granted through facial mask, high-flow nasal prongs or nasopharyngeal cannula. Facial mask can also be used to assist ventilation of the patient in case of hypercapnia (24).
If elective or urgent conversion to general anaesthesia is required (Table 2), the surgical team must be familiar with the crisis management protocol and have an effective communication (23). Emergency equipment must always be ready and the anaesthetist must be trained in lateral decubitus intubation skills. Double-lumen tube is preferred in case of urgent complication such as bleeding for minimal disturbance in the surgical site.

Full table
Surgical management
The aim of the aforementioned measures is to provide an operative field as similar as possible to general anaesthesia, where the mediastinum barely moves. After creating iatrogenic pneumothorax, and losing the negative intrathoracic pressure, the patient is likely to temporarily increase the rate and the depth of breathing while adapting to single-lung ventilation. Intercostal and vagal blocks should be performed as soon as possible. Then, the surgical site may be steady enough to gently perform anatomic resections if respiratory depth and rate remain normalized (12–20 bpm). These conditions are usually well kept for up to 2–3 h (24).
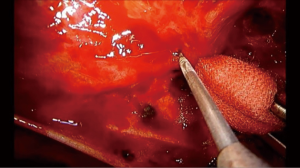
The correct assessment of any bleeding is paramount during NIVATS major procedures. If bleeding occurs, a sponge stick should be available to apply pressure immediately to control the haemorrhage. It is always important to remain calm and not to panic. If after several minutes the bleeding stops with compression, we can continue the lobectomy with no conversion to mechanical ventilation, performing extra careful dissection (Figure 6). However, any kind of major vessel injury may cause massive bleeding in case of an inadequate management of the defect that could be catastrophic in NIVATS patients. In this situation, early tracheal intubation and conversion to mechanical ventilation is recommended to preserve the safety of the patient.

When finishing the procedure, air leak test can be done by providing balloon-assisted ventilation through a well-sealed facial mask until underwater expansion is observed. If a tubeless option is preferred, drainage can be avoided in selected patients. Some groups use criteria proposed by Watanabe et al. (33), although with the above described insufflation technique air leak sealing test might not be needed. In absence of chest tube, routine ultrasound examination can contribute to the assessment of haemorrhage or pleural effusion (18). Complete tubeless approach lacking urinary or epidural catheter, central line and drainage allows for early oral intake, ambulation and discharge within 24 hours, enabling day surgery (12).
The avoidance of chest tube is more suitable for mediastinal tumor surgery, wedge resections or even anatomic single segmentectomies with no air leak checked at the end of the procedure (Figure 7). In case of lobectomy, the use of chest tube is recommended in the majority of the cases.

Nevertheless, NIVATS technique is constantly evolving, increasing the indications and pushing the limits of thoracic resections. A recent study showed that tracheal and carinal resections were also feasible in non-intubated patients with the benefit of avoiding challenges related to the endotracheal tube management during anastomosis without interfering with the surgical field (27). In this study the non-intubated thoracoscopic surgery technique for tracheal and carinal resections offered shorter operative times and potentially faster recovery than conventional intubated method.
Conclusions
Tubeless uniportal VATS has been feasible and safe in both minor and major anatomic lung resections when performed in precisely selected patients by experienced, proficient and cohesive thoracic surgery units. Intraoperative comfort and stability have been consistent enough, and the benefit for patients who otherwise would be rejected for surgical treatment has been obvious.
Although tubeless concept may decrease patient perturbation and translate into shorter hospital stay and faster recovery, larger prospective randomized trials are still needed to really elucidate its potential. Those results might encourage more teams to start a progressive, crisis management competent, learning curve.
The combination of tubeless NIVATS with uniportal approach is the least invasive way to perform surgery in our specialty to date, but new innovation opportunities such as the use of the subxiphoid approach, NOTES, or next generation single port robotic platform (35) must not be dismissed. The future of our speciality is in our hands as thoracic surgery innovators to find the least invasive approach possible for the benefit of our patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent:: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Gonzalez-Rivas D, Aymerich H, Bonome C, et al. From Open Operations to Nonintubated Uniportal Video-Assisted Thoracoscopic Lobectomy: Minimizing the Trauma to the Patient. Ann Thorac Surg 2015;100:2003-5. [Crossref] [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Double sleeve uniportal video-assisted thoracoscopic lobectomy for non-small cell lung cancer. Ann Cardiothorac Surg 2014;3. [PubMed]
- Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg 2004;78:1761-8. [Crossref] [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lung resection: a 3-year experience with 285 cases in a single institution. J Thorac Dis 2012;4:347-51. [PubMed]
- Liu J, Cui F, Pompeo E, et al. The impact of non-intubated versus intubated anaesthesia on early outcomes of video-assisted thoracoscopic anatomical resection in non-small-cell lung cancer: a propensity score matching analysis. Eur J Cardiothorac Surg 2016;50:920-5. [Crossref] [PubMed]
- Gonzalez-Rivas D, Bonome C, Fieira E, et al. Non-intubated video-assisted thoracoscopic lung resections: the future of thoracic surgery? Eur J Cardiothorac Surg 2016;49:721-31. [Crossref] [PubMed]
- Li S, Jiang L, Ang KL, et al. New tubeless video-assisted thoracoscopic surgery for small pulmonary nodules. Eur J Cardiothorac Surg 2017;51:689-93. [PubMed]
- Wang ML, Galvez C, Chen JS, et al. Non-intubated single-incision video-assisted thoracic surgery: a two-center cohort of 188 patients. J Thorac Dis 2017;9:2587-98. [Crossref] [PubMed]
- Al-Abdullatief M, Wahood A, Al-Shirawi N, et al. Awake anaesthesia for major thoracic surgical procedures: an observational study. Eur J Cardiothorac Surg 2007;32:346-50. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fernandez R, de la Torre M, et al. Single-port thoracoscopic lobectomy in a nonintubated patient: the least invasive procedure for major lung resection? Interact Cardiovasc Thorac Surg 2014;19:552-5. [Crossref] [PubMed]
- Shao W, Phan K, Guo X, et al. Non-intubated complete thoracoscopic bronchial sleeve resection for central lung cancer. J Thorac Dis 2014;6:1485-8. [PubMed]
- Li J, Liu J, Hamblin L, et al. Simple to simplest: the tubeless technique. J Thorac Dis 2017;9:222-4. [Crossref] [PubMed]
- Pompeo E, Sorge R, Akopov A, et al. Non-intubated thoracic surgery-A survey from the European Society of Thoracic Surgeons. Ann Transl Med 2015;3:37. [PubMed]
- Tacconi F, Pompeo E. Non-intubated video-assisted thoracic surgery: where does evidence stand? J Thorac Dis 2016;8:S364-75. [Crossref] [PubMed]
- Tacconi F, Pompeo E, Sellitri F, et al. Surgical stress hormones response is reduced after awake videothoracoscopy. Interact Cardiovasc Thorac Surg 2010;10:666-71. [Crossref] [PubMed]
- Vanni G, Tacconi F, Sellitri F, et al. Impact of awake videothoracoscopic surgery on postoperative lymphocyte responses. Ann Thorac Surg 2010;90:973-8. [Crossref] [PubMed]
- Guo Z, Yin W, Pan H, et al. Video-assisted thoracoscopic surgery segmentectomy by non-intubated or intubated anesthesia: a comparative analysis of short-term outcome. J Thorac Dis 2016;8:359-68. [Crossref] [PubMed]
- Cui F, Liu J, Li S, et al. Tubeless video-assisted thoracoscopic surgery (VATS) under non-intubated, intravenous anesthesia with spontaneous ventilation and no placement of chest tube postoperatively. J Thorac Dis 2016;8:2226-32. [Crossref] [PubMed]
- Gonzalez-Rivas D, Yang Y, Guido W, et al. Non-intubated (tubeless) uniportal video-assisted thoracoscopic lobectomy. Ann Cardiothorac Surg 2016;5:151-3. [Crossref] [PubMed]
- Pompeo E, Tacconi F, Mineo TC. Comparative results of non-resectional lung volume reduction performed by awake or non-awake anesthesia. Eur J Cardiothorac Surg 2011;39:e51-8. [Crossref] [PubMed]
- Galvez C, Navarro-Martinez J, Bolufer S, et al. Benefits of awake uniportal pulmonary resection in a patient with a previous contralateral lobectomy. Ann Transl Med 2014;2:93. [PubMed]
- Galvez C, Bolufer S, Navarro-Martinez J, et al. Awake uniportal video-assisted thoracoscopic metastasectomy after a nasopharyngeal carcinoma. J Thorac Cardiovasc Surg 2014;147:e24-6. [Crossref] [PubMed]
- Navarro-Martínez J, Gálvez C, Rivera-Cogollos MJ, et al. Intraoperative crisis resource management during a non-intubated video-assisted thoracoscopic surgery. Ann Transl Med 2015;3:111. [PubMed]
- Galvez C, Navarro-Martinez J, Bolufer S, et al. Nonintubated uniportal VATS pulmonary anatomical resections. J Vis Surg 2017;3:120. [Crossref] [PubMed]
- Peng G, Cui F, Ang KL, et al. Non-intubated combined with video-assisted thoracoscopic in carinal reconstruction. J Thorac Dis 2016;8:586-93. Erratum in: J Thorac Dis 2016;8:E641. [Crossref] [PubMed]
- Gonzalez-Rivas D. Uniportal thoracoscopic surgery: from medical thoracoscopy to non-intubated uniportal video-assisted major pulmonary resections. Ann Cardiothorac Surg 2016;5:85-91. [Crossref] [PubMed]
- Jiang L, Liu J, Gonzalez-Rivas D, et al. Thoracoscopic surgery for tracheal and carinal resection and reconstruction under spontaneous ventilation. J Thorac Cardiovasc Surg 2018;155:2746-54. [Crossref] [PubMed]
- Iwata Y, Hamai Y, Koyama T. Anesthetic management of nonintubated video-assisted thoracoscopic surgery using epidural anesthesia and dexmedetomidine in three patients with severe respiratory dysfunction. J Anesth 2016;30:324-7. [Crossref] [PubMed]
- Hung MH, Chan KC, Liu YJ, et al. Nonintubated thoracoscopic lobectomy for lung cancer using epidural anesthesia and intercostal blockade: a retrospective cohort study of 238 cases. Medicine (Baltimore) 2015;94. [Crossref] [PubMed]
- Lirio F, Galvez C, Bolufer S, et al. Intercostal multiple block with local anaesthesia under thoracoscopic view. Asvide 2018;5:695. Available online: http://www.asvide.com/article/view/26488
- Lirio F, Galvez C, Bolufer S, et al. Vagal block in the right lower paratracheal space. Asvide 2018;5:696. Available online: http://www.asvide.com/article/view/26490
- Lirio F, Galvez C, Bolufer S, et al. Bleeding control during non-intubated uniportal VATS left upper lobectomy. Asvide 2018;5:697. Available online: http://www.asvide.com/article/view/26492
- Watanabe A, Watanabe T, Ohsawa H, et al. Avoiding chest tube placement after video-assisted thoracoscopic wedge resection of the lung. Eur J Cardiothorac Surg 2004;25:872-6. [Crossref] [PubMed]
- Lirio F, Galvez C, Bolufer S, et al. Non-intubated uniportal VATS anatomic segmentectomy (S1). Asvide 2018;5:698. Available online: http://www.asvide.com/article/view/26493
- Gonzalez-Rivas D, Yang Y, Ng C. Advances in Uniportal Video-Assisted Thoracoscopic Surgery: Pushing the Envelope. Thorac Surg Clin 2016;26:187-201. [Crossref] [PubMed]