Postoperative morphine consumption and anaesthetic management of patients undergoing video-assisted or robotic-assisted lung resection: a prospective, propensity score-matched study
Introduction
Surgery is the gold standard treatment for early stage non-small cell lung cancer (NSCLC). Thoracotomy use is decreasing worldwide due to the emergence of minimally invasive approaches, such as video-assisted (VATS) and robot-assisted (RATS) thoracic surgeries. VATS is associated with lower postoperative pain and better quality of life compared to anterolateral muscle-sparing thoracotomy (1). Furthermore, several retrospective cohort studies and meta-analyses of non-randomised studies have shown significant reduction in the morbidity, especially due to respiratory complications (2-6). Despite these advantages of VATS, certain limitations of this technique, such as the steep learning curve, challenging hand-eye coordination, lack of instrument flexibility, two-dimensional vision, and some uncertainty regarding the quality of lymph node dissection that can be achieved using VATS, may still hinder its development (7).
RATS is an emerging option for managing patients requiring lung resection. Compared to VATS, RATS exhibited better procedure ergonomy and surgeon comfort. As RATS mimics open surgery, the learning curve for surgeons is possibly less steep than that for VATS. Moreover, the computer-assisted interface palliates hand tremor, thereby enhancing surgeon dexterity, and the magnified three-dimensional view improves visualisation of the operative field with the help of carbon-dioxide insufflation, potentially assisting more extensive dissection of the lymph nodes. Finally, articulated instruments allow seven degrees of motion as well as precise dissection and suturing in a confined operating space (8). However, whether this technologically expensive innovation provides superior outcomes remains unclear. The available literature suggests that RATS lobectomy is a feasible and safe technique that can achieve a short-term surgical efficacy equivalent to that achieved using VATS (9).
To our knowledge, no prospective study has evaluated the perioperative anaesthetic outcomes in patients undergoing VATS and RATS for lung resection. Thus, we conducted a study that aimed to compare the anaesthetic management and post-operative morphine consumption in patients undergoing lung cancer resection either with VATS or with RATS. Our primary objective was to compare the cumulative morphine use during the first two postoperative days. The secondary objective of our study was to compare the haemodynamic and respiratory changes during these two procedures.
Methods
This prospective, observational, comparative, single-centre study was conducted by the departments of anaesthesiology and thoracic surgery of the North University Hospital, Marseille, France. This research trial was approved by our Liberty and Informatics Committee (2016-18) and by the SFAR IRB (CERAR 00010254-2016-049). The study period ranged from January 2016 to March 2017. Written and oral information regarding the study purpose and procedures was given to all the patients before enrolling them, and written consents were collected. Consecutive patients undergoing lung resection for NSCLC suspicion were screened. Patients who required a thoracotomy or pre-resection mediastinoscopy, those who had previously undergone an ipsilateral thoracic surgery, those who reported chronic use of narcotics, and those with an altered mental status were excluded.
Surgical procedure
In accordance with the national and international guidelines, all operable patients referred to our high-volume academic institution with suspected clinical stage I NSCLC were offered a minimally invasive approach, either VATS or RATS (10,11). This study also included few patients who presented with benign conditions that necessitated lung resection. Two board-certified academic staff surgeons performed all the RATS procedures and performed or supervised all the VATS procedures. Patients were thus allocated either to the VATS or to the RATS group depending on the surgeon’s recruitment.
Our VATS program was initiated in the early 90’s (12). In 2010, we adopted the so-called totally thoracoscopic technique described by Gossot et al. (13). Accordingly, VATS pulmonary resections were performed using a 3-port technique, and no utility incision was used. The RATS procedures were performed with a da Vinci Surgical System Si (Intuitive Surgical Inc., Sunnyvale, CA, USA) available at our institution since Spring 2015. Among the four staff surgeons of the surgical team, two were previously identified to follow the step-by-step dedicated training. Both the RATS surgeons completed their clinical learning by 2015. The number of operations required was estimated to be 20, according to the literature (14). The 3-arm technique was used as routine, with an additional incision through which staplers were inserted and used by the assistant surgeon. Intrathoracic CO2 insufflation was used only in the RATS group.
On completion of the VATS or RATS pulmonary resection, the specimen was retrieved through a port site that was slightly enlarged, depending on the specimen size. The use of a rib spreader was not required for this task. In all cases, only one chest tube was placed through one of the port sites and was connected to a portable suction drainage system. Its removal was decided based on the standard guidelines, that is, no air leakage and output of <200 mL/day.
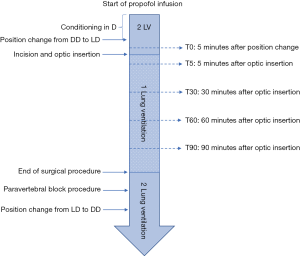
Full perioperative anaesthetic protocol and post-operative management is available on Figure S1.
Data management
All perioperative data were collected as shown in Figure S1 and File 1.
Data collection for pain evaluation
As per our primary objective, postoperative morphine consumption data were collected on day 1 and day 2, including the morphine used in the recovery room and surgical unit as well as the oral oxycodone (converted to intravenous morphine equivalent). Data collection was performed by a single investigator blinded to the surgical procedure. The visual analogue scale for pain (VAS) score was evaluated every 4 hours and reported by the nurse in charge of the patient. Nurses were not blinded to the surgical technique; however, they were not informed of the primary study objective. Minimal, mean, and maximal VAS scores on day 1 and day 2 were reported. The VAS score on coughing was evaluated once daily on days 1 and 2 after physiotherapist consultation by the same investigator.
Postoperative data collection
Postoperative complications were reported according to the modified Clavien-Dindo scale adapted to thoracic surgery during hospital stay and 28-day follow-up in cases of re-admission (15,16). Only the higher-grade complications were recorded. Postoperative complications were classified as minor according to the severity [grade I and II (including atrial fibrillation)] or major (grade III, IV, and V). Specific morphine consumption complications, such as nausea, vomiting, and urinary retention, were specifically reported. Durations of chest tube and hospital stay were recorded. Data regarding tumour grade, tumour histology, and lymph nodes were recorded from the definitive histological report after the surgery.
Sample size calculation
We hypothesised that the morphine consumption was lower after VATS than after RATS and aimed to detect a difference of 25% with a power of 0.9 and a 5% level significance. Under the assumption of a cumulated mean consumption of 30 (±14) mg morphine at day 2 for VATS, our calculation showed that 74 patients needed to be enrolled in each group for our primary end-point.
Considering the cardiac index (CI) comparison between the groups, we hypothesised a decrease in the RATS group compared to that in the VATS group and aimed to detect a difference of 15% with a power of 0.9 and a 5% level significance. Based on a local audit, we observed a mean CI of 2.5 (±0.6) L/min/m2 during VATS. We calculated that 46 patients were needed in each group for this secondary end-point.
Statistical analysis
The initial clinical characteristics were first described and compared according to the two groups of interest (VATS vs. RATS). Quantitative variables are presented as means (± SD) or medians (IQR) and compared using Student t-test when appropriate (Mann-Whitney test otherwise). Categorical variables are presented as numbers (percentages) and compared using Chi-Squared test when appropriate (Fisher test otherwise). Global outcomes (length of hospital stay, chest tubes duration, and incidence of complications) were analysed as per the intention to treat. Perioperative and post-operative data were described and compared for the two groups using the same indicators and statistical tests.
The statistical analyses of the primary outcome (morphine consumption at 48 hours) required a transformation of the dependent variable as the normality assumption could not be verified. The square root transformation method was chosen because of the existence of 0 in the morphine consumption, and this transformation allowed for non-rejection of the normality assumption. Univariate comparisons were performed between the transformed dependent variable and the characteristics potentially associated with morphine consumption (Student t-test was used for binary characteristics and Pearson correlation test was used for quantitative characteristics). Thereafter, a multivariate linear regression model was built, including the potential confounders of morphine consumption (use of continuous infusion of naropeine through the PVB and BMI); no data selection procedure based on statistical criteria was performed. To facilitate result interpretation by expressing the differences in milligrams of morphine, we presented a β coefficient using the square of the predicted values from the model using the transformed variable. To reduce the confounding by indication, we performed the same analyses using propensity score method (17), including baseline clinical variables (age, sex, and ASA score. Nearest neighbour matching without replacement was performed using a 1:1 ratio and a calliper equal to 0.3 of the standard deviation of logit of the propensity score.
The analysis of the evolution over time of secondary outcomes (perioperative data) was performed using multivariate linear mixed regression models. Multivariate models were proposed that included RATS (versus VATS), time, and basal value (T0 value) of the considered parameter. These final models incorporated β coefficients that represent a change in the dependent variable (I) when being exposed to RATS (reference: VATS), (II) when time increases (one additional measure), and (III) in reference to the basal value.
All analyses were performed using R software version 3.0.3 [R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: http://www.R-project.org/].
Results
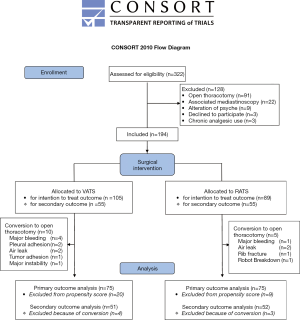
From January 2016 to March 2017, 332 patients who underwent lung resection were screened for study inclusion: three patients were unwilling to participate, and 125 did not meet the inclusion criteria. Finally, 194 patients were enrolled in the study, including 105 patients undergoing VATS and 89 patients undergoing RATS (Figure S2). The patients’ demographic data are presented in Table 1. No difference was found between the two groups with respect to preoperative characteristics (Table 1). We noted a difference in the mean comorbidity index of the VATS [5.7 (±4.0)] and the RATS [4.2 (±3.6)] groups (P=0.006); however, the mean thoracoscore of the VATS group [1.8 (±1.4)] was not significantly different from that of the RATS group [1.7 (±1.1)] (P=0.39). The use of continuous infusion from PVB was higher in the RATS group than in the VATS group (13.7% vs. 27.4%; P=0.02).
Full table
With respect to the intention-to-treat analysis, 10 (9.5%) and 5 (5.6%) patients required an open-procedure conversion in the VATS group and the RATS group (P=0.31), respectively (Table 1). No significant differences were noted in the mean duration of chest intubation in the VATS group [3.5 (3.0–5.0)] and the RATS group [3.5 (3.0–4.0)] days (P=0.25) and the average hospital stay duration of the two groups [VATS: 5.0 (4.0–7.0) vs. RATS 5.5 (3.5–8.0) days (P=0.07)]. There was no significant difference in the rate of minor complications between the groups. However, the rate of major complications was higher in the VATS group than in the RATS group (17.1% vs. 5.6%, P=0.01) (Table 1).
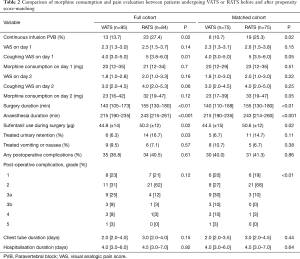
With respect to our primary outcome, after excluding the patients requiring open surgery conversion, 75 patients in each group were propensity score-matched for age, sex, and ASA status (Table 2). Using univariate analyses, the RATS group had higher morphine consumption during postoperative 48 hours than the VATS group [33.0 (19.3–46.5) vs. 23.0 (16.5–39.0) mg, P=0.05] (Figure 1). Linear regression revealed a mean difference β of [6.76 (0.32–13.26) mg; P=0.04] in morphine consumption, adjusted for BMI and on continuous infusion through the PVB, used in 13.7% and 27.4% of patients in the VATS and RATS groups, respectively (P=0.02). The use of continuous infusion was associated with reduced cumulated morphine use on day 2 [mean difference β of −9.65 (−17.8 to −1.6) mg; P=0.02].
Full table
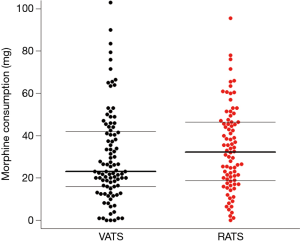
The surgical and anaesthetic durations were 142 (±51) vs. 161 (±45) min (P=0.01) and 215 (±44) vs. 244 (±45) min (P<0.01) in the VATS group and the RATS group, respectively. Sufentanil use was higher in the RATS group than in the VATS group [44.5 (±15) vs. 50.6 (±12) µg, P=0.02]. Urinary retention was higher in the RATS group (16.7% vs. 6.3%, P=0.03). Reported incidence rates of nausea and vomiting were similar in the two groups (Table 2). Graded complications analysis found a higher rate for grade 2 complications in the RATS group and a higher rate of major complications in the VATS group (P=0.01). Detailed distribution of the complications is presented in Table 2.
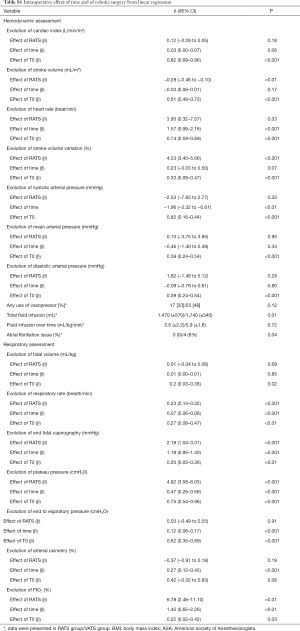
Perioperative haemodynamic and respiratory data were fully assessed for 103 patients (51 VATS and 52 RATS) (in total online: http://jtd.amegroups.com/public/system/jtd/supp-jtd.2018.05.179-1.pdf) (Table S1). No differences were found in the demographic features of the two groups (Table S1). Respiratory and haemodynamic data were similar at T0, except for PEP [6 (±1) vs. 5 (±1) cmH2O in the VATS and the RATS group, respectively, P=0.02]. Anaesthetic management was similar for the groups (Table S1). No significant differences were reported between BIS, TOF count, and central temperature between the two groups over time.
Full table
The influence of time and robotic procedure, adjusted on baseline (T0), on the evolution of the haemodynamic and respiratory variables is shown in Table S1. The kinetics of the measured and predicted haemodynamic and respiratory variables are represented in Figures 2,3.
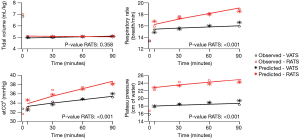
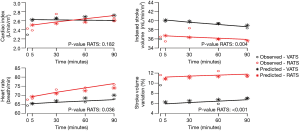
With respect to haemodynamics, the use of vasopressors was similar in both the groups. Total volume of crystalloid was higher in the RATS group than in the VATS group [1,470 (±570) vs. 1,740 (±540) mL; P=0.01]; however, it was comparable when adjusted to the anaesthesia duration and patient weight [5.6 (±2.2) vs. 5.9 (±1.8) mL/kg/h; P=0.72]. The CI did not differ between the groups; no influence of time or RATS was determined. The heart rate increased with an effect of RATS [β =3.95 (0.32 to 7.57); P=0.03], while a decrease in the indexed stroke volume was affected by RATS [β = −0.28 (−0.46 to −0.10); P<0.01] (Table S1).
With respect to respiratory assessment, we found an effect of RATS on plateau pressure increase [β =4.82 (3.58 to 6.05); P<0.01] and on end-tidal expiratory CO2 increase [β =2.18 (1.04 to 3.31); P=0.001] despite a significant increase in the respiratory rate [β =0.23 (0.14 to 0.32); P<0.01] (Table S1).
Discussion
To our knowledge, our study is the first prospective comparison of RATS and VATS. RATS was associated with an increased cumulated consumption of morphine on day 2. We also found haemodynamic and respiratory differences, suggesting significant effects of RATS.
The choice of the morphine consumption as a primary outcome seemed relevant because it is an indicator of postoperative pain, is an independent factor for postoperative complications, and is a quality marker of ERAS procedure (18,19). The increased use of morphine in the RATS group could be explained by the forced rib traction exerted during RATS without force-return to the surgeon, creating potential intercostal nerves lesions, and the addition of one utility incision in RATS compared to that in VATS. In a retrospective study, Kwon et al. found no differences in the morphine consumption and pain between patient undergoing RATS or VATS as compared to those undergoing open thoracotomy (20). Their study showed pain risk factors, such as age, sex, and pre-operative morphine use. In our study, we used age and sex for elaborating a propensity score matching. The patients with preoperative morphine use were excluded from our study.
Regarding the haemodynamic and respiratory assessment, our analyses suggests a significant role of CO2 insufflation during RATS with a mean pressure of 8 mmHg. The heart rate increased during the RATS procedure, associated with decreased stroke volume; however, the cardiac index remained unchanged. This may indicate that the venous return was more impaired in patients undergoing RATS than in those undergoing VATS. It is noteworthy that stroke volume variations increased during RATS. This could be attributable to inadequate fluid resuscitation, impaired right ventricular function, or a non-relevant measurement technique. The elevation of plateau pressure associated with insufflation pressure of CO2 suggests increased heart-lung interaction in RATS. However, we cannot rule out the usefulness of pulse contour analysis in these patients. Effect of CO2 insufflation has been widely described during abdominal celioscopic surgery on haemodynamic and respiratory impairment and increased pain (21). Even if the effect of CO2 reabsorption may be comparable, effects of CO2 reabsorption during RATS need to be confirmed in future studies.
Regarding the rate of major complications and open-surgery conversion, RATS appears to be a safer procedure. The number of minor complications was higher in the RATS group. However, there was an increase in the post-operative atrial fibrillation in the RATS group, if the open-procedure conversions were excluded. This could be secondary to pericardial irritation with CO2. Velez-Cubian et al. described atrial fibrillation as a frequent complication following RATS. An association between postoperative atrial fibrillation and impaired outcome was reported (22).
Our study has certain limitations. First, although this was a prospective study, our subjects were not randomised. We attempted to minimise this bias by using propensity score-matched analyses. However, undetermined variables may have influenced the differences determined between the RATS and VATS groups. For instance, in our institution, RATS is a recent procedure, while VATS is a standard treatment. However, our findings underline the need for a randomised clinical trial that assesses the relevance of RATS compared to VATS. Another issue is that the use of PVB with or without continuous infusion of an anaesthetic solution differed between the two groups. This is attributable to the gradual arrival of the “one shot” PVB technique in our centre that aims to decrease catheter use promoted by our fast recovery protocol. However, our statistical analyses considered this difference by means of multivariate regression models. In fact, we showed that continuous infusion was associated with lower morphine use. Thus, this reinforces our result, underlining the higher morphine consumption associated with the RATS group. VATS and RATS were performed by different surgeons, leading to potential bias. This is explained by the shared robotic material with other surgery units and the minimal learning curve for RATS (20 to 30 procedures) that explained the need of concentrate the use other 2 of the 4 broad-certified surgeons of our center (14). Finally, the use of clearsightTM for haemodynamic measurement is questionable. Though this device has been validated in cardiac surgery against thermodilution, it has not been validated during one-lung ventilation (23). However, the use of this volume clamp monitoring technique on the ipsilateral side of surgery is believed not to interfere with any arterial compression or intrathoracic manipulation (24).
In conclusion, our results show that RATS is associated with a higher postoperative morphine consumption. In addition, it highlights the haemodynamic and respiratory effects associated with RATS. Finally, in our cohort, the RATS procedure appeared safer than VATS.
File 1 Intraoperative anaesthesia protocol
Surgical procedure
In accordance with the national and international guidelines, all operable patients referred to our high-volume academic institution with suspected clinical stage I NSCLC were offered a minimally invasive approach, either VATS or RATS (10,11) This study also included few patients who presented with benign conditions that necessitated lung resection. Two board-certified academic staff surgeons performed all the RATS procedures and performed or supervised all the VATS procedures. Patients were thus allocated either to the VATS or to the RATS group depending on the surgeon’s recruitment.
Our VATS program was initiated in the early 90’s (12). In 2010, we adopted the so-called totally thoracoscopic technique described by Gossot et al. (13). Accordingly, VATS pulmonary resections were performed using a 3-port technique, and no utility incision was used. The RATS procedures were performed with a da Vinci Surgical System Si (Intuitive Surgical Inc., Sunnyvale, CA, USA) available at our institution since Spring 2015. Among the four staff surgeons of the surgical team, two were previously identified to follow the step-by-step dedicated training. Both the RATS surgeons completed their clinical learning by 2015. The number of operations required was estimated to be 20, according to the literature (14). The 3-arm technique was used as routine, with an additional incision through which staplers were inserted and used by the assistant surgeon. Intrathoracic CO2 insufflation was used only in the RATS group.
On completion of the VATS or RATS pulmonary resection, the specimen was retrieved through a port site that was slightly enlarged, depending on the specimen size. The use of a rib spreader was not required for this task. In all cases, only one chest tube was placed through one of the port sites and was connected to a portable suction drainage system. Its removal was decided based on the standard guidelines, that is, no air leakage and output of <200 mL/day.
Specific anaesthetic procedures
Perioperative anaesthetic protocol
Anaesthesia management was performed according to our protocol. For induction, propofol was used at an initial effect-site target concentration between 4 and 6 µg/mL using a target-controlled infusion device with a built-in modified Schneider model (OrchestraTM Base Primea; Fresenius Vial, Brézins, France). Sufentanil was administered as a single bolus (0.1 to 0.3 µg/kg). Cisatracurium was administered as the initial bolus (0.15 to 0.2 mg/kg). The trachea was intubated with a double lumen tube of CarlensTM type.
For anaesthesia maintenance, a continuous infusion of propofol with site effect target concentration between 3 and 5 µg/mL was used. The anaesthesia depth was measured using the BIS (BIS™, Philips M1034A, Eindhoven, The Netherlands) with a target between 40 and 60. The muscle relaxant effect was measured using TOF monitoring with a target at 0–1 count on the ulnar nerve. All patients were monitored using an intranasal thermic probe.
Surgery was performed with the patient in the lateral decubitus position, depending on the surgery side. After incision, one-lung ventilation was initiated. Mechanical ventilation during one lung ventilation was set for a tidal volume of 5 mL/kg of the ideal weight, positive end-expiratory pressure of 5 cmH2O, and oxygen fraction of 0.6 to 1 for an oxygen saturation of ≥95%.
Paravertebral block protocol
All patients received a unilateral paravertebral block (PVB) established by the in-charge anaesthesiologist at the end of the procedure using either anatomic, using the loss-of-resistance technique, or ultrasound-guided, using the in-plane approach, aiming the 5th thoracic vertebral level. For improving rehabilitation, our protocol recommends a single shot; however, the use of continuous infusion was at the anaesthesiologist’s discretion. A 10-mL bolus of 10 mg/mL xylocaine with 0.005 mg/mL adrenaline was used to check for the absence of intravasculaire infusion. Thereafter, a 20-mL bolus of 5% ropivacaine was administered as a single shot (in the operative room) or before the onset of continuous infusion of 2% ropivacaine (in the postoperative room) in case of catheter use after its placement was evaluated using chest radiography with opacification. Patients were monitored for at least 30 minutes for signs of local anaesthetic toxicity and efficiency of PVB using cold tests. When catheters were used, the infusion protocol involved the use of ropivacaine 2% with degressive infusion posology other time (24 mg/hour on day 1 to 12 mg/hour on day 5). Catheters were removed at the team’s discretion between day 3 and day 5.
Postoperative analgesic protocol
Thirty minutes before sedation interruption, all the patients received intravenous paracetamol and ketoprofen; for prevention of nausea and vomiting, 2.5 mg droperidol was administered. Pain was evaluated in the post-operative recovery room using a visual analogic scale (VAS) pain score. Titrated morphine was infused, if required, to achieve a VAS score <30. In the surgical unit, all the patients received a combination of intravenous paracetamol and ketoprofen as well as a patient-controlled analgesia pump (PCA) that was initiated in the recovery room. The morphine dilution was 1 mg/mL with the addition of 0.05 mg/mL droperidol. Boli were administered by patient: 1 mg every 7 minutes or 1.5 mg every 7 minutes for patients weighing >80 kg. Oral analgesics and narcotics were used if the venous lines were removed. Oral narcotics included oxycodone medication. VAS was evaluated every 4 to 6 hours and recorded. Oral narcotics were prescribed to patients whenever the VAS was >30 mm. An investigator collected data regarding narcotic consumption and VAS score during coughing for 48 h after the surgery.
Post-operative management
Post-operative management was performed according to our local protocol. All the patients underwent a standardised fast rehabilitation protocol, including early post-operative oral nutrition and armchair positioning (within 6 hours). ERAS protocol included a systematic daily respiratory rehabilitation, early ambulation with physiotherapist and oral transition of medication at day 1 if possible (adequate pain evaluation and absence of refractory vomiting). Chest tubes were removed as soon as possible after the post-operative chest radiography excluded exhaustive pneumothorax or pleural effusion.
Data collection
Pre-operative data collection
Demographic characteristics were extracted from the electronic medical chart. Age, sex, height, weight, side of surgery, and type of lung reduction were recorded. The ASA, Lee, and Apfel scores were computed. Respiratory function was assessed using pulmonary function testing, including forced expiratory volume in 1 second and the carbon monoxide diffusion capacity adjusted for the alveolar volume. The Thoracoscore and associated morbidity index were extracted from the EPITHOR database (15).
Smoking status was recorded, including the total tobacco use calculation and weaning. Patients were considered as weaned if they had stopped smoking for more than 4 weeks, as defined in the French guidelines for maximal clinical benefit (16).
Perioperative data collection
Patients were gradually included for perioperative data collection, depending on availability of the haemodynamic monitoring system that was shared with others surgical units. Patients included in the haemodynamic evaluation were monitored using a ClearsightTM system with adapted EV 1000 monitoring station (Edwards Lifesciences, Irvine, California, USA). An appropriately sized sensor was placed around the second phalange of the second finger of the ipsilateral hand of the surgery to prevent arterial compression due to lateral decubitus. The anaesthesiologist in charge of the patient was blinded from the EV 1000 monitoring station that was placed on a parametric screen instead of on a monitoring screen, and data were collected using USB Key after the surgical procedure. Systolic, mean, and diastolic blood pressure, heart rate and cardiac index were assessed. Respiratory data were recorded from the ventilator readings (Primus, Dragër Medical, Lübeck, Germany), including the tidal volume, respiratory rate, positive expiratory pressure, oxygen fraction, oxygen arterial saturation, end-tidal CO2, and tele-inspiratory plateau pressure. Data of train-of-four (TOF), bi-spectral index (BIS), and body temperature were collected. The insufflation pressure of carbon dioxide used during RATS and incidence of per-operative cardiac rhythm abnormalities were also recorded.
At the end of the procedure, the time from skin incision to skin closure (including the time taken for chest tubes fixation) was calculated. After PVB was established the time of anaesthesia and total consumption of propofol, sufentanil, cisatracurium, and fluid infusion were reported. In cases where a vasopressor was used, the type and dosage administered were noted.
Acknowledgements
Authors want to specially thank Drs. Vincent Boustière, Romain Ronflé, Bruno Pastène, Aurelia Hili, Luca Servan, David Fiocchi, Antoine Tilmont and all the anaesthesiologist nurse team for their help for the peroperative management of the patients.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This research trial was approved by our Liberty and Informatics Committee (2016-18) and by the SFAR IRB (CERAR 00010254-2016-049). The study period ranged from January 2016 to March 2017. Written and oral information regarding the study purpose and procedures was given to all the patients before enrolling them, and written consents were collected.
References
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Cao C, Manganas C, Ang SC, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small cell lung cancer: a meta-analysis of propensity score-matched patients. Interact Cardiovasc Thorac Surg 2013;16:244-9. [Crossref] [PubMed]
- Jeon JH, Kang CH, Kim HS, et al. Video-assisted thoracoscopic lobectomy in non-small-cell lung cancer patients with chronic obstructive pulmonary disease is associated with lower pulmonary complications than open lobectomy: a propensity score-matched analysis. Eur J Cardiothorac Surg 2014;45:640-5. [Crossref] [PubMed]
- Boffa DJ, Dhamija A, Kosinski AS, et al. Fewer complications result from a video-assisted approach to anatomic resection of clinical stage I lung cancer. J Thorac Cardiovasc Surg 2014;148:637-43. [Crossref] [PubMed]
- Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Pagès PB, Delpy JP, Orsini B, et al. Propensity Score Analysis Comparing Videothoracoscopic Lobectomy With Thoracotomy: A French Nationwide Study. Ann Thorac Surg 2016;101:1370-8. [Crossref] [PubMed]
- Zhang W, Wei Y, Jiang H, et al. Thoracotomy is better than thoracoscopic lobectomy in the lymph node dissection of lung cancer: a systematic review and meta-analysis. World J Surg Oncol 2016;14:290. [Crossref] [PubMed]
- Watson TJ. Robotic esophagectomy: is it an advance and what is the future? Ann Thorac Surg 2008;85:S757-9. [Crossref] [PubMed]
- Wei S, Chen M, Chen N, et al. Feasibility and safety of robot-assisted thoracic surgery for lung lobectomy in patients with non-small cell lung cancer: a systematic review and meta-analysis. World J Surg Oncol 2017;15:98. [Crossref] [PubMed]
- Thomas P, Dahan M, Riquet M, et al. Practical issues in the surgical treatment of non-small cell lung cancer. Recommendations from the French Society of Thoracic and Cardiovascular Surgery. Rev Mal Respir 2008;25:1031-6. [Crossref] [PubMed]
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:7S-37S.
- Giudicelli R, Thomas P, Lonjon T, et al. Major pulmonary resection by video assisted mini-thoracotomy. Initial experience in 35 patients. Eur J Cardiothorac Surg 1994;8:254-8. [Crossref] [PubMed]
- Gossot D, Zaimi R, Fournel L, et al. Totally thoracoscopic pulmonary anatomic segmentectomies: technical considerations. J Thorac Dis 2013;5:S200-6. [PubMed]
- Veronesi G. Robotic lobectomy and segmentectomy for lung cancer: results and operating technique. J Thorac Dis 2015;7:S122-30. [PubMed]
- Seely AJ, Ivanovic J, Threader J, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg 2010;90:936-42; discussion 942. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Rosenbaum PR, Rubin DB. The Central Role of the Propensity Score in Observational Studies for Causal Effects. 1983 [cited 2017 Oct 26]; Available online: https://dash.harvard.edu/handle/1/3382855
- Feldheiser A, Aziz O, Baldini G, et al. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand 2016;60:289-334. [Crossref] [PubMed]
- Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain 2016;17:131-57. [Crossref] [PubMed]
- Kwon ST, Zhao L, Reddy RM, et al. Evaluation of acute and chronic pain outcomes after robotic, video-assisted thoracoscopic surgery, or open anatomic pulmonary resection. J Thorac Cardiovasc Surg 2017;154:652-9.e1. [Crossref] [PubMed]
- Özdemir-van Brunschot DM, van Laarhoven KC, Scheffer GJ, et al. What is the evidence for the use of low-pressure pneumoperitoneum? A systematic review. Surg Endosc 2016;30:2049-65. [Crossref] [PubMed]
- Velez-Cubian FO, Ng EP, Fontaine JP, et al. Robotic-Assisted Videothoracoscopic Surgery of the Lung. Cancer Control 2015;22:314-25. [Crossref] [PubMed]
- Broch O, Renner J, Gruenewald M, et al. A comparison of the Nexfin® and transcardiopulmonary thermodilution to estimate cardiac output during coronary artery surgery. Anaesthesia 2012;67:377-83. [Crossref] [PubMed]
- Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol 2005;90:437-46. [Crossref] [PubMed]


