Radical treatment of primary type B aortic dissection or after thoracic endovascular aortic repair to manage disseminated intravascular coagulation
Introduction
Thoracic endovascular aneurysm repair (TEVAR) is now a preferred therapeutic approach for acute complicated Stanford type B aortic dissection and specific chronic aortic dissection, which has replaced traditional open surgery and has obvious advantages (1). Evidence has also demonstrated long-term benefits of TEVAR over optimal medical treatment (2). The main approach of TEVAR treatment for type B aortic dissection is generally to seal the proximal entry tear only to achieve true lumen (TL) expansion, promote false lumen thrombosis (FLT) and avoid paraplegia caused by spinal cord ischemia. However, approximately 30% of patients simultaneously presenting with additional distal tears will need reinterventions due to the enlargement of the false lumen (FL) by continuous blood flow perfusion (3). Although the incidence of further expansion with uncovered distal entry tears was reported to be less than 20% after TEVAR in follow-up, failure of FLT is still an ongoing Achilles’ heel of TEVAR in the treatment for type B aortic dissection (4). Occasionally, the incomplete thrombosed FL with persistent flow may consume large amounts of coagulation material and fibrinogen, resulting in disseminated intravascular coagulation (DIC) and other complications (5). DIC is an uncommon and fatal postoperative complication of TEVAR and has been associated with endoleak after endovascular intervention repairs (5-11). Patients often exhibit unexplained subcutaneous ecchymosis after aortic dissection diagnosis or TEVAR, and the blood tests show decreased fibrinogen and elevated D-dimer. It was proposed that properly selective exclusion of the remaining distal tears is a feasible approach for promoting FLT post-TEVAR (12). The aim of this study is to present two cases of DIC caused by Stanford type B aortic dissection and described the radical endovascular repair treatment method to manage DIC.
Surgical techniques
Case 1
A 73-year-old man complaining of back pain with chest wall hematoma for 6 days was admitted to our hospital. He received TEVAR for Stanford type B aortic dissection 8 years ago. The latest follow-up computed tomographic angiography (CTA) showed no obvious abnormalities, except for a slightly delayed dilation of the FL observed 2 years ago. Other histories included high blood pressure (HBP), coronary heart disease (CHD, receiving PCI treatment 4 months ago) and atrial fibrillation for more than 10 years. Physical examination found a large area of hematomas within the right chest wall accompanied by tenderness. Laboratory examination showed the fibrinogen level was 99 mg/dL (normal range, 200–400 mg/dL), D-dimer was 11,410 ng/mL (normal range, 20–800 ng/mL) and platelets were 91×109/L (normal range, 125–350×109/L), while the liver function test results were all in the normal threshold. CTA demonstrated no significant endoleak in the stent segment but showed contrast perfusion of the FL through multiple tears near the visceral artery area (Figure 1).
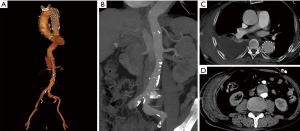
Due to the local puncture drainage of non-coagulated blood, this patient was highly suspected of progressive bleeding (Figure 2), and thus emergency endovascular reintervention was carried out. Briefly, after the bilateral femoral artery was preloaded with double Perclose ProGlide (Abbott Laboratories, Abbott Park, IL, USA), distal tears were found in the distal portion of the descending aorta above the celiac trunk near the previous stent-graft, as well as in the abdominal aorta below the left renal artery and in the bilateral iliac arteries through the pre-procedure angiogram. The right renal artery originated from the FL. A 45 mm × 45 mm × 200 mm C-TAG stent-graft (W.L. Gore and Associates, Flagstaff, AZ, USA) was deployed with the proximal end partially overlapped with the previous stent through right femoral access to seal the distal tears above the celiac trunk. A 28 mm × 28 mm × 80 mm Endurant stent-graft (Medtronic, Minneapolis, MN, USA) was placed from the left renal artery level to the abdominal aortic bifurcation to seal all multiple distal tears in this segment. Crossing the FL, the right renal artery was cannulated and followed with a 7 F × 70 cm sheath (Cook Medical, Bloomington, IL, USA) from the left femoral artery, and a 6 mm × 100 mm Viabahn (W.L. Gore and Associates, Flagstaff, AZ, USA) was advanced into the right renal artery from the TL and deployed. Then, the MPA catheter and glide wire were cannulated into the FL through the distal tears in the iliac artery and exchanged into a Lunderquist wire (Cook Medical, Bloomington, IL, USA) with an 18F Gore sheath from the left side. A 26 mm × 26 mm × 100 mm C-TAG stent-graft was deployed in the FL to induce FLT formation and exclude the perivisceral tears from outside. Afterwards, 6 Nest coils (Cook Medical, Bloomington, IL, USA) were placed on the top of the C-TAG densely, as a “mushroom head”, to promote thrombosis formation in the FL. Furthermore, a 16 mm × 120 mm Excluder iliac extension (W.L. Gore and Associates, Flagstaff, AZ, USA) and a 10 mm×50 mm Viabahn were deployed in the left and right iliac arteries, respectively. The final angiogram demonstrated good position of all the grafts and no endoleak during the delayed phase. Laboratory evaluation demonstrated that the levels of fibrinogen and platelets increased to normal 2 and 5 days after the procedure, respectively. In addition, the D-dimer level also decreased to 4,220 ng/mL when discharged. The patient recovered well and was discharged 1 week postoperatively. At the 6-month follow-up, the patient had no symptoms of DIC. CTA demonstrated total thrombosis of the FL without endoleak (Figure 3).
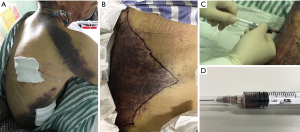
Case 2
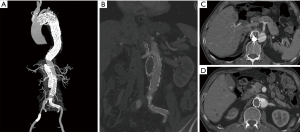
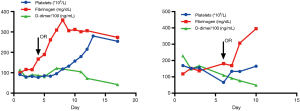
A 74-year-old man presenting with back pain for 1 week was referred to our emergency room. The admission CTA revealed that the primary tear of the dissection was located just distal to the left subclavian artery. All the visceral arteries were patent. Among them, the celiac trunk, the superior mesenteric and the left renal arteries originated from the TL, while the right renal artery was from both the TL and FL. The patient denied a history of HBP and CHD. Physical examination showed that a subcutaneous hematoma was on the right chest wall with an area of approximately 20 cm × 30 cm. Laboratory evaluation demonstrated a platelet count of 69×109/L (normal range, 125–350×109/L), fibrinogen level of 118 mg/dL (normal range, 200–400 mg/dL), and D-dimer level of 22,910 ng/mL (normal range, 20–800 ng/mL). CTA showed a partial thrombosed FL filled with contrast. Based on these findings, the patient was diagnosed with DIC secondary to the aortic dissection. Given the high risk of open surgery in the setting of DIC, the patient was offered radical endovascular intervention repair. In addition to excluding the primary entry tears by a Captivia stent-graft (32 mm × 32 mm × 200 mm, Medtronic, Minneapolis, MN, USA), an Endurant cuff (28 mm × 28 mm × 80 mm) was also used to cover the tears of the abdominal aorta below the left renal artery. A 6 mm × 50 mm Viabahn for the right renal artery and a 13 mm × 50 mm Viabahn for the right iliac artery were deployed to seal all distal tears. A 34 mm × 34 mm × 150 mm C-TAG stent-graft was deployed in the FL to promote thrombosis formation. The fibrinogen increased to a normal range of 307 mg/dL after 2 days. The last laboratory assessment in wards demonstrated that the platelet count increased to 165×109/L, fibrinogen increased to a normal value of 395 mg/dL and D-dimer decreased to 4,930 ng/mL. The patient recovered uneventfully and was discharged 5 days after the procedure. The changing trend of the typical DIC indexes peri-operatively is shown in the Figure 4. At the 3-month follow-up after the intervention, the patient was also free of any symptoms of DIC, and the CTA revealed full expansion of the TL, completed thrombosis formation in the FL and no endoleak.
Discussion
These two patients were diagnosed with DIC secondary to type B aortic dissection after admission to our emergency department. They presented with unexplained chest ecchymosis associated with subcutaneous hematoma. CTA confirmed that the hematoma was within the chest wall and multiple tears were positioned in the aorta which allowed blood flow to continuously perfuse the FL. After physical examination and laboratory tests, it was found that the DIC indicators of fibrinogen and platelets had declined to different degrees, while D-dimer increased. It was worthy to point out that the follow-up of fibrinogen, D-dimer and platelet count values are necessary to identify the DIC complication for patients with aortic dissection.
DIC is a severe condition involving excessive consumption of platelets and coagulation material with resultant bleeding, which is usually caused by many risk factors, such as infection, surgery, cancer, liver disease, pregnancy complication and others (13). Given no history of such diseases in these two cases, we postulated that DIC was secondary to aortic dissection. However, the exact mechanism underlying this complication has not been fully elucidated yet. Possible pathophysiological mechanism may be that turbulent flow in FL expose denuded endothelium and tissue factor, leading to activation of coagulation factors, chronic consumption of clotting factors, and simultaneous fibrinolysis of the clots (5). In addition, the thrombus formation and dissolution constantly occurring in the FL can also lead to the consumption and liberation of coagulated material. Consequently, abnormal diagnostic indexes of DIC and relevant symptoms can be observed. For example, subcutaneous bleeding on the chest wall was displayed in these two patients.
Given that the DIC complication mainly resulted from the consumption of fibrinogen and coagulation material inside the FL, we decided to perform radical endovascular repair to occlude all the tears for these two patients. Relative studies on sealing distal tears or radical surgery for dissection are rare since this therapeutic strategy is not yet included in the guidelines. Our technique was proposed to cover not only the primary tear but also all the remaining distal tears after initial TEVAR or for the primary type B dissection, so that the blood flow inside the FL could be cut off, which prevented further continuous consumption of coagulation components and platelets. In some cases, due to the rapid expansion of the distal FL after TEVAR, reinterventions were also applied to repair the secondary or distal tears. According to the location of the orifices, different devices, such as bare stent, Viabahn, Amplatzer plugs, multilayer flow modulator, coils and others, were selected. The “Knickerbocker” and “candy-plug” techniques were also reported to manage the patients with persistent FL flow and aneurysm enlargement (14,15). In our study, a C-TAG stent-graft was inserted in the FL not only to prevent coils from being rushed away but also to occlude the orifices from inside of the FL. In addition, the number of coils used in the procedure was decreased. In these two cases, with multiple stent-grafts coordinating to exclude all the tears, the DIC conditions were controlled, and no recurrent DIC occurred in the follow-up session. Furthermore, FLT formation and no endoleak were demonstrated in both cases.
DIC is a severe and rare complication secondary to de novo type B aortic dissection or after TEVAR, which is due to the continuous consumption of the coagulation factors in the FL during the constant process of thrombosis formation and dialysis. Based on the satisfactory clinical outcomes of these two cases, we concluded that the radical endovascular repair to exclude the primary and all the secondary/distal tears is a feasible approach to induce FLT formation and thus to manage DIC effectively.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (Grant number: 81570438) and Shanghai science and technology commission Innovation Fund (Grant number: 18441902400).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Nauta FJ, Trimarchi S, Kamman AV, et al. Update in the management of type B aortic dissection. Vasc Med 2016;21:251-63. [Crossref] [PubMed]
- Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv 2013;6:407-16. [Crossref] [PubMed]
- Nienaber CA, Rousseau H, Eggebrecht H, et al. Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation 2009;120:2519-28. [Crossref] [PubMed]
- Andacheh ID, Donayre C, Othman F, et al. Patient outcomes and thoracic aortic volume and morphologic changes following thoracic endovascular aortic repair in patients with complicated chronic type B aortic dissection. J Vasc Surg 2012;56:644-50; discussion 50. [Crossref] [PubMed]
- Nienaber JJ, Duncan AA, Oderich GS, et al. Operative and nonoperative management of chronic disseminated intravascular coagulation due to persistent aortic endoleak. J Vasc Surg 2014;59:1426-9. [Crossref] [PubMed]
- Cross KS, Bouchier-Hayes D, Leahy AL. Consumptive coagulopathy following endovascular stent repair of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2000;19:94-5. [Crossref] [PubMed]
- Higashiura W, Kichikawa K, Sakaguchi S, et al. Deteriorating consumptive coagulopathy with type III endoleak following endovascular repair for abdominal aortic aneurysm associated with liver cirrhosis. J Endovasc Ther 2007;14:421-5. [Crossref] [PubMed]
- Ohara N, Miyata T, Oshiro H, et al. Adverse outcome following transfemoral endovascular stent-graft repair of an abdominal aortic aneurysm in a patient with severe liver dysfunction: Report of a case. Surg Today 2000;30:764-7. [Crossref] [PubMed]
- Patel AS, Bell R, Hunt BJ, et al. Disseminated intravascular coagulation after endovascular aneurysm repair: resolution after aortic banding. J Vasc Surg 2009;49:1046-9. [Crossref] [PubMed]
- Pesteil F, Labrousse L, Chevreuil C, et al. Preoperative hepatic insufficiency and type III endoleak: a confirmed potential fatal association following endovascular treatment. Interact Cardiovasc Thorac Surg 2008;7:285-7. [Crossref] [PubMed]
- Mendes BC, Oderich GS, Erben Y, et al. False Lumen Embolization to Treat Disseminated Intravascular Coagulation After Thoracic Endovascular Aortic Repair of Type B Aortic Dissection. J Endovasc Ther 2015;22:938-41. [Crossref] [PubMed]
- Wojtaszek M, Wnuk E, Maciag R, et al. Promoting False-Lumen Thrombosis after Thoracic Endovascular Aneurysm Repair in Type B Aortic Dissection by Selectively Excluding False-Lumen Distal Entry Tears. J Vasc Interv Radiol 2017;28:168-75. [Crossref] [PubMed]
- Keo HH, Diehm N, Baumgartner I, et al. Disseminated Intravascular Coagulopathy Caused by Endoleak Type I: Successful Treatment by Endovascular Stent-graft Extension. EJVES Extra 2006;12:68-70. [Crossref]
- Kolbel T, Carpenter SW, Lohrenz C, et al. Addressing persistent false lumen flow in chronic aortic dissection: the knickerbocker technique. J Endovasc Ther 2014;21:117-22. [Crossref] [PubMed]
- Kolbel T, Lohrenz C, Kieback A, et al. Distal false lumen occlusion in aortic dissection with a homemade extra-large vascular plug: the candy-plug technique. J Endovasc Ther 2013;20:484-9. [Crossref] [PubMed]


