The role of postoperative radiotherapy for radically resected esophageal squamous cell carcinoma: a systemic review and meta-analysis
Introduction
Esophagus cancer (EC) is the eighth most common cancer worldwide and the sixth leading cause of cancer death (1,2). Esophageal adenocarcinoma (EAC) predominates in western countries, while esophageal squamous cell carcinoma (ESCC) is the most common histological type in Asian countries. Surgical management is still considered as the mainstay of treatment for all resectable cases. However, surgery alone (S alone) showed poor long-term outcomes, and the 5-year survival rate was rarely >30% even after curative resection (3,4). Neoadjuvant chemoradiation followed by surgery has been a standard treatment in western countries (5-8). However, many Asian patients still choose surgery as their initial therapy, especially in China.
Postoperative radiotherapy (PORT) is not recommended by the current National Comprehensive Cancer Network (NCCN) guidelines for patients who underwent radical resection (9). However, many patients developed local recurrence or distant metastasis (10). A number of studies have investigated whether PORT leads to improved cure rates compared with S alone, but individual reports have been conflicting (11-29). A meta-analysis of five randomized controlled trials (RCTs) performed by Malthaner et al. found no benefit from PORT in patients with ESCC (30). However, all included RCTs were designed more than 20 years ago, employed a conventional two-dimensional radiotherapy (2D-RT) technique, three of them were small with fewer than 50 patients in the treatment group, and two of them included data of palliative resection. Since this meta-analysis, data from one recently published RCT (11) and some retrospective studies including several large retrospective studies (17-25) have demonstrated a potential benefit from PORT in patients with resectable ESCC. Therefore, it is necessary to reevaluate the value of PORT for ESCC. In the present study, we performed a systematic review and meta-analysis of currently available evidences to further determine whether PORT improves survival compared with S alone in radically resected ESCC.
Methods
This meta-analysis was conducted in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) criteria (31).
Literature search strategy
PubMed, EMBASE, Web of Science, and the Cochrane Library were searched for the available articles published before September 1, 2017, using the strategy as follows: ((esophageal cancer [Title/Abstract]) OR (esophageal carcinoma [Title/Abstract])) AND ((postoperative [Title/Abstract]) OR (adjuvant [Title/Abstract])) AND ((radiotherapy [Title/Abstract]) OR (radiation therapy [Title/Abstract]) OR (chemoradiotherapy [Title/Abstract])). Only studies in English were considered. All published papers with available full texts were retrieved. Reference lists of retrieved articles were manually scanned for relevant additional studies missed by the electronic search. The study did not involve any experiment on humans or animals, thus ethical approval was not necessary.
Inclusion and exclusion criteria
Studies were included if they met the following criteria: (I) types of studies. RCT, or prospective or retrospective cohort study; (II) types of participants. Participants with a histopathological diagnosis of ESCC and resectable disease; (III) types of interventions. Patients with surgery as their initial treatment and compared patients who received radical resection with or without PORT; (IV) outcome: reported survival [overall survival (OS) and/or disease-free survival (DFS)] data. If multiple articles covered the same study population, the study with the most recent and complete survival data was used. Studies were excluded if any of the following criteria were applied: (I) letters, editorials, case reports, and reviews; (II) survival data could not be extracted from the literature.
Data extraction
The data were extracted by two investigators independently, and the consensus was reached in case of any discrepancy for all the data. The following data were extracted from each study: first author, years of publication, duration of the study, country of origin, numbers of patients (with and without PORT), study design, time-to-event data (OS, DFS), locoregional recurrence and distant hematogenous metastases data, and occurrence of grade 3–4 adverse events. In case that studies did not report sufficient data, authors of those studies were contacted for further information by Email if possible.
Quality assessment
The Newcastle-Ottawa Quality Assessment Scale (NOS) was used to assess the quality of retrospective studies (32). The NOS comprises of three items: patient selection, comparability of the study groups, and assessment of outcomes. The quality of each cohort study was scored on a scale ranging from 0 to 9 by two independent researchers. Six stars or greater was considered to be sufficiently high-quality studies.
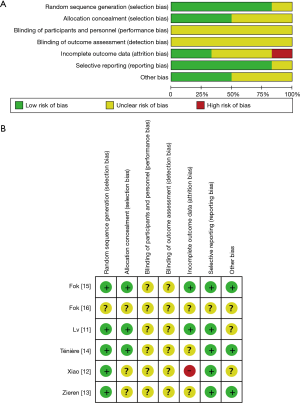
The methodological quality of RCTs was assessed by Cochrane risk of bias tool (33), which consists of the following five domains: sequence generation, allocation concealment, blinding, incomplete data, and selective reporting. An RCT was finally rated as “low risk of bias” (all key domains indicated as low risk), “high risk of bias” (one or more key domains indicated as high risk), and “unclear risk of bias”.
Statistical analysis
Statistical analysis was performed using the software Review Manager 5.3 (Cochrane Collaboration, Oxford, UK) and STATA MP14.0 (Stata Corporation, College station, TX, USA). Because the median survival or survival rates at a specific point in time were not expected to be reliable surrogate measures for the pooled survival analysis, hazard ratios (HRs) and their 95% CIs were used as summary statistics for OS in the present meta-analysis. Crude HRs with 95% CIs were either extracted directly from the original reports or calculated by the Kaplan-Meier curves based on the methods of Parmar et al. (34) and Tierney et al. (35). A statistical test for heterogeneity was performed by the Chi-square (χ2) and I-square (I2) test with significance set at P<0.10 and/or I2>50%. If significant heterogeneity existed, a random-effects analysis model was used; otherwise, a fixed-effects model was used. In addition, we conducted subgroup and meta-regression analysis to search for the source of heterogeneity. The stability of the pooled results was evaluated by a sensitivity analysis in which the data of an individual study was removed each time. The funnel plot, Begg’s test (36), and the Egger’s linear regression test (37) were performed to investigate any potential publication bias. If evidence of publication bias was observed, the trim and fill method (38) was applied to correct the bias. A P value <0.05 was considered to be statistically significant.
Results
Literature search results and characteristics of included studies
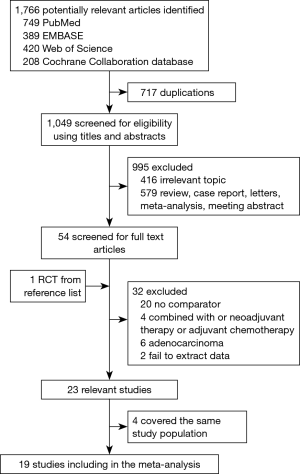
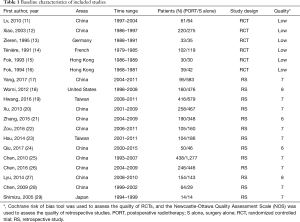
The literature search and study selection procedures are shown in Figure 1. The initial search from the electronic database retrieved 1,766 articles. After removing the duplicates, 1,049 citations were identified. Of these, 995 were excluded through an abstract review. The remaining 54 articles were screened through a full-text review for further eligibility. Because two Taiwan Cancer Registry-based articles, two articles of Xiao et al., and three articles of Chen et al. covered the same study population, four of them were excluded, and three articles (12,14,25) with the most recent and complete survival data were retained. Finally, 6 RCTs and 13 retrospective studies assessing 8,198 patients (2,779 patients receiving PORT and 5,419 patients receiving S alone) were included in the meta-analysis. For six included RCTs, five of them (12-16) were the same with that enrolled in the previous meta-analysis performed by Malthaner et al. (30), and the remaining one was new eligible (11). Two RCTs (11,15) included data of palliative resection and radical resection, but only data of radical resection were extracted. Characteristics of the eligible studies were summarized in Table 1.
Full table
Assessment of included studies
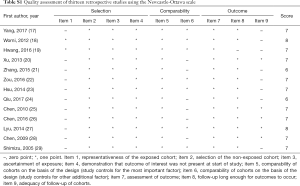
The two researchers showed good consistency in assessing the study quality of nineteen included studies (Table 1). All of the retrospective studies demonstrated a score of ≥6 (Table S1). The qualities of the included RCTs were generally low. One RCT were considered to be in “high risk”, and the remaining RCTs were classified as “unclear” with respect to the risk of bias (Figure S1).
Full table
Primary outcomes: OS and DFS
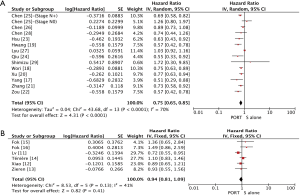
Multivariable adjusted HRs for OS were used to calculate pooled HR for 4 of 19 studies (22,24-25,28), and univariable adjusted HRs were used for others. Multivariable adjusted HRs for DFS were used to calculate pooled HR for 2 of 5 studies (22,23), and univariable adjusted HRs were used for others. Significantly statistical difference was observed between PORT and S alone groups in a pooled analysis of OS for 5,657 patients from all included retrospective studies (HR =0.75, 95% CI: 0.65–0.85, Pheterogeneity<0.0001), but not for 1,050 patients from all included RCTs (HR =0.94, 95% CI: 0.81–1.09, Pheterogeneity=0.13) (Figure 2). PORT was associated with significantly improved DFS compared to S alone both for retrospective studies (5 studies with 1,378 patients; HR =0.72, 95% CI: 0.62–0.83, Pheterogeneity=0.12) and RCTs (3 studies with 414 patients; HR =0.69, 95% CI: 0.54–0.88, Pheterogeneity=0.69) (Figure 3).
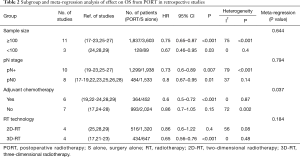
Subgroup and meta-regression analyses of OS in retrospective studies are detailed in Table 2. Except subgroup of PORT with 2D-RT (HR =0.86, 95% CI: 0.6–1.22, Pheterogeneity=0.08), and PORT without chemotherapy (HR =0.86, 95% CI: 0.7–1.05, Pheterogeneity=0.002), PORT was associated with significantly improved OS in sample size ≥100 (HR =0.75, 95% CI: 0.65–0.87, Pheterogeneity<0.001), sample size <100 (HR =0.67, 95% CI: 0.46–0.95, Pheterogeneity=0.4), patients with lymph node-positive (pN+) (HR =0.73, 95% CI: 0.6–0.89, Pheterogeneity<0.001), patients with lymph node-negative (pN0) (HR =0.8, 95% CI: 0.67–0.95, Pheterogeneity=0.14), PORT with 3D-RT (HR =0.65, 95% CI: 0.56–0.76, Pheterogeneity=0.48), and PORT with chemotherapy (HR =0.6, 95% CI: 0.5–0.72, Pheterogeneity=0.87), respectively. Significant difference of OS was also observed between PORT and S alone for patients with pT2–3N0M0 (4 studies with 653 patients; HR =0.74, 95% CI: 0.6–0.91, Pheterogeneity=0.11) and patients with R0 resection (9 studies with 3,867 patients; HR =0.73, 95% CI: 0.66–0.8, Pheterogeneity=0.18). Results of meta-regression analysis demonstrated that PORT with/without chemotherapy was the evident contributor of heterogeneity (P=0.037) (Table 2). Subgroup analysis of OS in RCTs was not performed due to lack of number of studies.
Full table
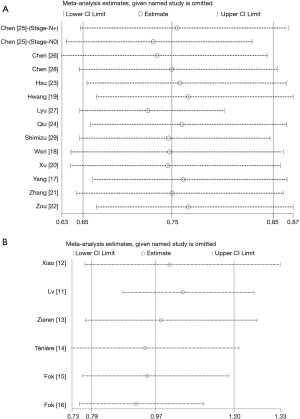
Sensitivity analyses were carried out to assess whether individual studies influenced the results in retrospective studies and RCTs, respectively. When individual studies were removed one at a time from the analyses for OS, the corresponding pooled HRs were not markedly altered by any single study (HR lies between 0.72 and 0.76 in retrospective studies and between 0.92 and 1.1 in RCTs), confirming the stability of the presented results (Figure S2).
Secondary outcomes: locoregional recurrence and distant hematogenous metastasis
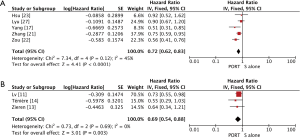
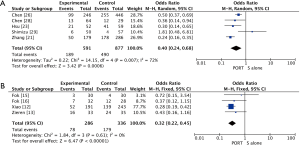
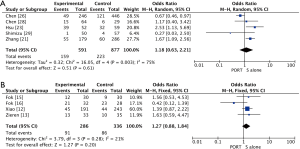
The pooled results showed that PORT significantly decreased the risk of locoregional recurrence compared to S alone in either retrospective studies (5 studies with 1,468 patients; OR =0.40, 95% CI: 0.24–0.68, Pheterogeneity=0.007) or RCTs (4 studies with 622 patients; OR =0.32, 95% CI: 0.22–0.45, Pheterogeneity=0.61) (Figure 4). There was no significant difference of distant hematogenous metastases between PORT and S alone both for retrospective studies (5 studies with 1,468 patients; OR =1.18, 95% CI: 0.63–2.21, Pheterogeneity=0.003) and RCTs (4 studies with 622 patients; OR =1.27, 95% CI: 0.88–1.84, Pheterogeneity=0.28) (Figure S3).
Toxicity
Toxicities were largely underreported in the included publications. Grade 3 or 4 hematological toxicities were reported in five studies (2–18.1%) (20-22,24,29). Grade 3 or 4 radiation pneumonitis and esophagitis were observed in five studies (1.9–6.6%) (17,20-22,24) and in three studies (2.9–9.5%) (17,21,22), respectively. Grade 3 or 4 late toxicities were reported in four studies (12,16,17,21). One studies (12) reported non-cancerous pericardial effusion or pleural effusion (3.2%) and radiation lung fibrosis (2.3%) in PORT group. Anastomotic stenosis (reported in two studies) was similar between PORT and S alone group (OR =1.94, 95% CI: 0.8–4.69) (12,21). One studies (12) reported gastrointestinal bleeding in PORT group (2%), and another (21) reported that either in PORT (2%) or S alone group (2%). Only one studies (16) reported anastomotic leakage in PORT group (2%).
Assessment of publication bias
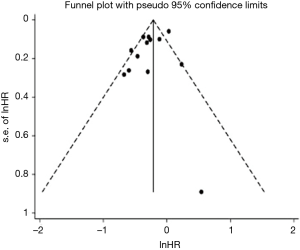
Publication bias in terms of OS was assessed in retrospective studies, but not done in RCTs due to the lack of number of studies. The funnel plot is shown in Figure S4. Although the Begg’s test results indicated no publication bias (P=0.511), Egger’s test suggested a borderline significant probability of publications bias (P=0.084). However, the trim and fill method demonstrated that no missing studies were detected, indicating that our results were reliable.
Discussion
Neoadjuvant chemoradiotherapy (CRT) remains to be the standard treatment modality for locally advanced EC based on the results of the CROSS trial (7). The CROSS study demonstrated a 14% increase in 5-year OS for patients with EC (both squamous cell carcinoma and adenocarcinoma) treated with neoadjuvant CRT compared with surgery alone (7). In the latest network meta-analysis conducted by Montagnani et al., 25 trials were included, neoadjuvant CRT was associated with the most robust survival advantage across different multimodality treatment options, but adjuvant CRT was associated with a non-significant benefit (39). However, we have to be confronted with is that the initial treatment for majority of patients’ trends to be surgery in China for various reasons. Although postoperative multidisciplinary treatment including RT and CRT has been vigorously implemented, there are no current practical guidelines suggesting postoperative treatments, possibly due to the absence of a large randomized trial or a high-quality meta-analysis demonstrating its survival benefits.
To our knowledge, this is the first meta-analysis to evaluate the role of PORT in radical resected ESCC. The meta-analysis enrolled a total of 19 studies (including 6 RCTs and 13 retrospective studies) with 8,198 patients. The primary findings were that PORT provided significant OS benefit compared with S alone in retrospective studies, but not in RCTs; PORT was associated with significantly improved DFS, obvious reduction in the risk of locoregional recurrence and a similar incidence of distant hematogenous metastasis when compared to S alone in either retrospective studies or RCTs. There was significant heterogeneity for OS in retrospective studies. Based on subgroup and meta-regression analysis of OS in retrospective studies, PORT with/without chemotherapy was identified as an evident contributor of heterogeneity. The sensitivity analysis for OS revealed that the corresponding pooled HRs were robust when individual studies were removed one at a time from the analyses.
There may be several possible explanations why RCTs failed to show OS benefit with the use of PORT. Firstly, 2D-RT technique was used in all included RCTs. Compared with 2D-RT technique, 3D-RT delivered a high dose to the tumor target volume while potentially minimizing the radiation dose to the organ at risk. Most of individual studies of PORT using 3D-RT showed consistent OS benefit compared to S alone (17,21-23), while the results of studies using 2D-RT were various. In current meta-analysis, PORT significantly improved OS for PORT using 3D-RT, but not for that using 2D-RT when compared to S alone. Secondly, adjuvant chemotherapy was not used in combination with PORT in most of included RCTs (12-16). Only one included RCT (11) used PORT with chemotherapy and showed significant improved OS. OS benefit from PORT with adjuvant chemotherapy was also reported in several retrospective studies (19,22,23). A meta-analysis comparing surgery followed by adjuvant CRT to surgery without adjuvant CRT (non-CRT) for resectable esophageal carcinoma concluded that CRT could gain a survival benefit (40). In the present analysis, PORT showed significant improvement of OS compared with S alone in subgroup of PORT with chemotherapy, but not in subgroup of PORT alone which accounted for the most of heterogeneity of the treatment effect on OS. Thirdly, data from included RCTs were of low quality, and the sample size of them were small (three of them were with fewer than 50 patients in PORT group). Thus, it might be underpowered to detect the difference in OS.
The survival effect of PORT for different lymph node status remains undetermined. Most of the retrospective studies showed the survival benefit of PORT in patients with pN+ compared with S alone (19-23,25,26). Results from one RCT performed by Xiao et al. (12) showed that the 1-, 3-, and 5-year survival rates for patients with pN+ were 69.7%, 24.7%, and 14.7% in S alone group and 72.3%, 38.2%, and 29.2% in PORT group, respectively. These differences nearly reached statistical significance (P=0.0698). In line with these individual studies, PORT could gain significant OS and DFS compared with S alone for patients with pN+ in the present analysis. However, there was still lack of consensus on the value of PORT for patients with pN0 or pT2–3N0. Two RCTs (12,14) using 2D-RT technique showed no survival improvement of PORT for patients with pN0 or pT2–3N0. However, results from one more recent large retrospective studies using 3D-RT showed that PORT was strongly associated with an improved OS and DFS in pT3N0M0 ESCC patients (17). In current meta-analysis, significant OS and DFS benefit from PORT were observed for patients with pN0 or pT2–3N0. Whether PORT using 3D-RT technique is critical in improving survival for patients with pN0 or pT2–3N0 needs further investigation.
There are several limitations in our meta-analysis. Firstly, all of included RCTs were of low quality which might be underpowered to detect the difference in OS. Secondly, significant heterogeneity was seen in pooled-analysis of OS (I2=70%) in retrospective studies. By using subgroup and meta-regression analysis, PORT with/without chemotherapy was identified as evident contributor of heterogeneity. Thirdly, a few HRs were not directly reported in the texts, and hence calculated from the Kaplan-Meier curve. This may result in bias and error. Fourthly, several individual studies did not report resection status, and a part of patients with R1 resection should be contained which might be a confounding factor. However, subgroup analysis of R0 resection showed a survival benefit from PORT (HR =0.73, 95% CI: 0.66–0.8). Finally, majority of included studies were performed in China, thus, extending the conclusions to other regions should be discreet. Other confounding factors may also affect the survival, such as radiation doses, tumor location, pathological grade, and operation type. However, we could not conduct a subgroup analysis of that due to lack of detailed data or number of studies.
Conclusions
The present study shows that PORT can improve DFS and decrease risk of locoregional recurrence in patients with radically resected ESCC, and PORT using 3D-RT or in combination with chemotherapy is likely to be more useful. Further well-designed, prospective studies are needed to confirm the effect of PORT on OS.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study did not involve any experiment on humans or animals, thus ethical approval was not necessary.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Kumagai K, Rouvelas I, Tsai JA, et al. Meta-analysis of postoperative morbidity and perioperative mortality in patients receiving neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal and gastro-oesophageal junctional cancers. Br J Surg 2014;101:321-38. [Crossref] [PubMed]
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400-12. [Crossref] [PubMed]
- Hsu PK, Wu YC, Chou TY, et al. Comparison of the 6th and 7th editions of the American Joint Committee on Cancer tumor-node-metastasis staging system in patients with resected esophageal carcinoma. Ann Thorac Surg 2010;89:1024-31.
- Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014;32:2416-22. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 2005;6:659-68. [Crossref] [PubMed]
- National Comprehensive Cancer Network Guidelines. Esophageal and Esophagogastric Junction Cancers version 3.2015. Available online: http://www.spg.pt/wp-content/uploads/Guidelines/NCCN/2015%20esophageal%20(1).pdf
- Chen J, Pan J, Zheng X, et al. Number and location of positive nodes, postoperative radiotherapy, and survival after esophagectomy with three-field lymph node dissection for thoracic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2012;82:475-82. [Crossref] [PubMed]
- Lv J, Cao XF, Zhu B, et al. Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J Gastroenterol 2010;16:1649-54. [Crossref] [PubMed]
- Xiao ZF, Yang ZY, Liang J, et al. Value of radiotherapy after radical surgery for esophageal carcinoma: a report of 495 patients. Ann Thorac Surg 2003;75:331-6. [Crossref] [PubMed]
- Zieren HU, Muller JM, Jacobi CA, et al. Adjuvant postoperative radiation therapy after curative resection of squamous cell carcinoma of the thoracic esophagus: a prospective randomized study. World J Surg 1995;19:444-9. [Crossref] [PubMed]
- Ténière P, Hay JM, Fingerhut A, et al. Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle and lower esophagus as shown by a multicenter controlled trial. French University Association for Surgical Research. Surg Gynecol Obstet 1991;173:123-30. [PubMed]
- Fok M, Sham JS, Choy D, et al. Postoperative radiotherapy for carcinoma of the esophagus: a prospective, randomized controlled study. Surgery 1993;113:138-47. [PubMed]
- Fok M, McShane J, Law SY, et al. Prospective randomised study in the treatment of oesophageal carcinoma. Asian J Surg 1994;17:223-9.
- Yang J, Zhang W, Xiao Z, et al. The Impact of Postoperative Conformal Radiotherapy after Radical Surgery on Survival and Recurrence in Pathologic T3N0M0 Esophageal Carcinoma: A Propensity Score-Matched Analysis. J Thorac Oncol 2017;12:1143-51. [Crossref] [PubMed]
- Worni M, Martin J, Gloor B, et al. Does surgery improve outcomes for esophageal squamous cell carcinoma? An analysis using the surveillance epidemiology and end results registry from 1998 to 2008. J Am Coll Surg 2012;215:643-51. [Crossref] [PubMed]
- Hwang JY, Chen HS, Hsu PK, et al. A Propensity-matched Analysis Comparing Survival After Esophagectomy Followed by Adjuvant Chemoradiation to Surgery Alone for Esophageal Squamous Cell Carcinoma. Ann Surg 2016;264:100-6. [Crossref] [PubMed]
- Xu Y, Liu J, X. D, et al. Prognostic impact of postoperative radiation in patients undergoing radical esophagectomy for pathologic lymph node positive esophageal cancer. Radiat Oncol 2013;8:116. [Crossref] [PubMed]
- Zhang W, Liu X, Xiao Z, et al. Postoperative intensity-modulated radiotherapy improved survival in lymph node-positive or stage III thoracic esophageal squamous cell carcinoma. Oncol Res Treat 2015;38:97-102. [Crossref] [PubMed]
- Zou B, Pang J, Liu Y, et al. Postoperative chemoradiotherapy improves survival in patients with stage II-III esophageal squamous cell carcinoma: An analysis of clinical outcomes. Thorac Cancer 2016;7:515-21. [Crossref] [PubMed]
- Hsu PK, Huang CS, Wang BY, et al. Survival benefits of postoperative chemoradiation for lymph node-positive esophageal squamous cell carcinoma. Ann Thorac Surg 2014;97:1734-41. [Crossref] [PubMed]
- Qiu B, Li J, Wang B, et al. Adjuvant Therapy for a Microscopically Incomplete Resection Margin after an Esophagectomy for Esophageal Squamous Cell Carcinoma. J Cancer 2017;8:249-57. [Crossref] [PubMed]
- Chen J, Zhu J, Pan J, et al. Postoperative radiotherapy improved survival of poor prognostic squamous cell carcinoma esophagus. Ann Thorac Surg 2010;90:435-42. [Crossref] [PubMed]
- Chen SB, Weng HR, Wang G, et al. The impact of adjuvant radiotherapy on radically resected T3 esophageal squamous cell carcinoma. J Cancer Res Clin Oncol 2016;142:277-86. [Crossref] [PubMed]
- Lyu X, Huang J, Mao Y, et al. Adjuvant chemotherapy after esophagectomy: is there a role in the treatment of the lymph node positive thoracic esophageal squamous cell carcinoma? J Surg Oncol 2014;110:864-8. [Crossref] [PubMed]
- Chen G, Wang Z, Liu XY, et al. Clinical study of modified Ivor-Lewis esophagectomy plus adjuvant radiotherapy for local control of stage IIA squamous cell carcinoma in the mid-thoracic esophagus. Eur J Cardiothorac Surg 2009;35:1-7. [Crossref] [PubMed]
- Shimizu K, Hihara J, Yoshida K, et al. Clinical evaluation of low-dose cisplatin and 5-fluorouracil as adjuvant chemoradiotherapy for advanced squamous cell carcinoma of the esophagus. Hiroshima J Med Sci 2005;54:67-71. [PubMed]
- Malthaner RA, Wong RK, Rumble RB, et al. Neoadjuvant or adjuvant therapy for resectable esophageal cancer: a systematic review and meta-analysis. BMC Med 2004;2:35. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [Crossref] [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [Crossref] [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455-63. [Crossref] [PubMed]
- Montagnani F, Fornaro L, Frumento P, et al. Multimodality treatment of locally advanced squamous cell carcinoma of the oesophagus: A comprehensive review and network meta-analysis. Crit Rev Oncol Hematol 2017;114:24-32. [Crossref] [PubMed]
- Zheng B, Zheng W, Zhu Y, et al. Role of adjuvant chemoradiotherapy in treatment of resectable esophageal carcinoma: a meta-analysis. Chin Med J (Engl) 2013;126:1178-82. [PubMed]