Long-term outcomes and prognostic factors of patients with surgically treated pulmonary atypical carcinoid tumors: our institutional experience with 68 patients
Introduction
Pulmonary atypical carcinoid (AC) tumors are rare intermediate-grade malignant tumors which are derived from neuroendocrine Kulchitsky cells of the bronchopulmonary mucosa and submucosal glands and comprise from 10% to 35% of all pulmonary carcinoid tumors (1). Based on the latest World Health Organization (WHO) classification of bronchopulmonary carcinoids, AC are defined as ≥2 mitoses but <10 mitoses per 2 mm2, coagulative necrosis, or both (2). Although surgical resection is the preferred treatment for AC, the appropriate management of AC remains controversial and the prognosis is still uncertain.
In our study, we aimed to analyze clinicopathologic features, long-term outcomes, surgical strategy and prognostic factors of AC patients in our institution.
Methods
This retrospective study involved 68 consecutive patients surgically treated for AC at Cancer Hospital, Chinese Academy of Medical Science between March 1999 and May 2013. The standard preoperative evaluation included pulmonary function test, electrocardiogram, standard posterolateral chest films, computed tomography of chest and brain, abdominal ultrasound, bone scintigraphy and positron emission tomography (PET) scan when necessary. All patients had a preoperative tracheobronchial endoscopy. Data on age, gender, presenting symptoms, history of smoking, tumor size and location, pathological stage, immunohistochemical expression, lymph node status, type of surgical procedure, postoperative complications and long-term survival were analyzed. The tumors were staged according to the 8th edition of the tumor, node, and metastasis (TNM) classification of lung cancer. This study was approved by the ethics committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College.
Following surgery, the patients were followed every 3 months in the first year and every 6 months thereafter. The follow-up was performed at clinic visits and by telephone or mail.
Statistical analysis was performed using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). Relapse-free survival (RFS) was considered to be the period between surgery and the date of the recurrence, and overall survival (OS) was considered to be the period between surgery and the date of the last follow-up or death. The Kaplan-Meier method was used for calculating survival rates and the log-rank test was used for determining significance of the differences in RFS and OS. All univariate and multivariate analyses for RFS and OS were performed using the Cox regression model. Factors that showed statistically significant association with survival in univariate analysis were included into multivariate Cox proportional hazards model. Statistical significance was accepted as P<0.05 throughout the study.
Results
From 1999 to 2013, there were 68 patients diagnosed with primary pulmonary AC at our institution. There were 55 males (80.9%) and 13 females (19.1%), with male to female ratio of 4.2:1. The mean age at diagnosis was 55 years, ranging from 30 to 78 years. Forty-four patients (64.7%) had previous or current tobacco use.
The majority of the patients (n=47; 69.1%) were symptomatic at presentation: 37 patients (54.4%) presented with cough; 19 patients (27.9%) presented with hemoptysis; 11 patients (16.2%) presented with dyspnea; 10 patients (14.7%) presented with chest/back discomfort or pain; 7 patients (10.3%) presented with a variety of other symptoms and signs, including fever, weakness, weight loss, night sweats. Only 1 patient (1.5%) presented with carcinoid syndrome including wheezing, diarrhea, and flushing. Twenty-one patients (30.9%) were asymptomatic at presentation. Thirty-one patients had comorbid diseases, 11 patients with hypertension, 8 patients with diabetes mellitus, 3 patients with hypertension and diabetes mellitus, 3 patients with chronic pulmonary disease, 2 patients with coronary artery disease, 2 patients with hypertension and coronary artery disease, 1 patient with connective tissue disease and 1 patient with cerebrovascular disease.
In our series, 28 patients (41.2%) had a centrally located tumor and 40 patients (58.8%) presented with peripheral tumor localization. The most common site was the left upper lobe (n=24; 35.3%). The tumor was located in the right lung in 32 patients (47.1%), and left lung in 36 patients (52.9%).
Surgical procedures were carried out for the 68 patients included 49 lobectomies, 10 pneumonectomies, 6 bilobectomies, 2 sleeve lobectomies, and 1 wedge resection. Systematic mediastinal lymph node dissection was added in all cases. Surgery was the only treatment in 31 patients, whereas 37 other patients also received chemotherapy and/or radiation treatment. The chemotherapy regimens administered included etoposide plus cisplatin, paclitaxel plus cisplatin, navelbine plus cisplatin, and irinotecan plus cisplatin. The radiotherapy dosage in these patients was 50–60 Gy. One patient had a liver metastasis and was treated by hepatic artery interventional therapy. No operative or postoperative mortality was observed. Postoperative complications were observed in 5 patients (7.4%), including arrhythmia (n=2; 2.9%), persistent air-leakage (n=2; 2.9%), pneumonia (n=1; 1.5%).
According to the 8th edition of the International Classification of TNM, the postoperative TNM stage was as follows: 22 patients (32.4%) were in stage I, 27 (39.7%) were in stage II, 19 (27.9%) were in stage III. There were 25 T1 tumors (36.8%), 25 T2 tumors (36.8%), 14 T3 tumors (20.6%) and 4 T4 tumors (5.9%). According to N status, there were 38 N0 patients (55.8%), 15 N1 patients (22.1%) and 15 N2 patients (22.1%). Information on immunohistochemical staining was available in 57 of the 68 patients (83.8%) in this study. Synaptophysin was positive in 45 specimens (78.9%), chromogranin A in 44 specimens (77.2%), CD56 in 37 specimens (64.9%) and TTF1 in 19 specimens (33.3%).
Patients were classified according to Ki-67 levels in three subgroups (>0 and <5%, >5% and <10%, and ≥10%). Among 48 patients tested for Ki-67 expression, 21 (43.8%) had a Ki-67 level less than 5% whereas 12 (25.0%) had a Ki-67 level of at least 10%; the other 15 patients (31.3%) had Ki-67 levels of 5 to 10%, respectively.
At the time of last follow-up, 29 of the 68 patients (42.6%) were dead in the overall cohort and 20 recurrences (29.4%) were noticed. Sixteen patients died from disease recurrence, whereas 13 patients died due to unrelated causes and without signs of recurrence. No patients were lost to follow-up. The median follow-up period was 48 months (range, 7–170 months). The overall RFS was 70.6%. The most common recurrence was in the lung (35.0%) followed by mediastinal lymph node (25.0%), bones (15.0%), liver (15.0%), brain (10.0%).
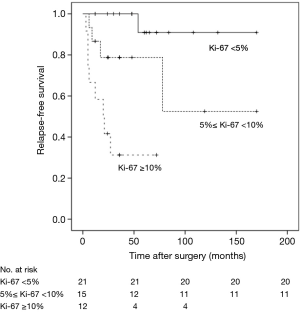
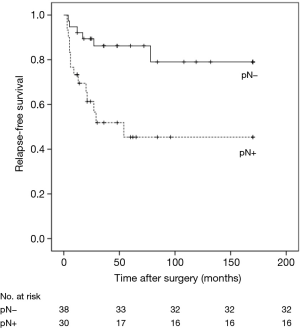
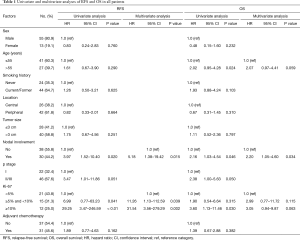
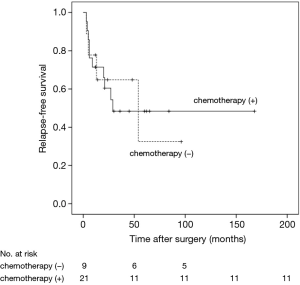
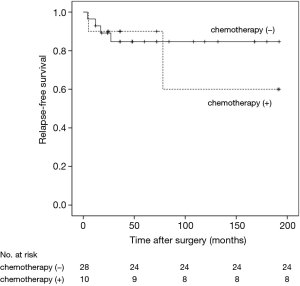
RFS was significantly worsened by increasing Ki-67 index (Ki-67 <5% vs. ≥5% and <10%, HR =6.99, 95% CI: 0.77–63.23, P=0.041; Ki-67 <5% vs. ≥10%, HR =29.25, 95% CI: 3.47–246.59, P<0.01) (Figure 1) and lymph-nodes involvement (HR =3.97; 95% CI: 1.52–10.40, P=0.020) (Figure 2). RFS of the three subgroups with different Ki-67 levels (>0 and <5%, ≥5% and <10%, and ≥10%) was 95.2%, 73.3% and 33.3%, respectively. According to lymph node status, RFS of N0 patients, N1 patients and N2 patients was 84.2%, 73.3% and 33.3%, respectively. In multivariate analysis, increasing Ki-67 index (Ki-67 <5% vs. ≥5% and <10%, HR =11.26, 95% CI: 1.13–112.59, P=0.039; Ki-67 <5% vs. ≥10%, HR =31.54, 95% CI: 3.56–279.29, P=0.002) and lymph-nodes involvement (HR =5.18, 95% CI: 1.38–19.42, P=0.015) were still poor prognostic factors for RFS (Table 1). For those patients with lymph-nodes involvement, no statistically significant difference was found in RFS between those who received adjuvant chemotherapy treatment after resection and those who had operation alone (HR =0.97, 95% CI: 0.30–3.09, P=0.957) (Figure 3). In the measure of RFS of N0 patients who had operation alone and those who received adjuvant chemotherapy, no significant difference was found between the two groups (HR =1.40, 95% CI: 0.26–7.64, P=0.698) (Figure 4).
Full table
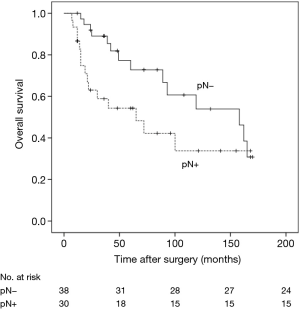
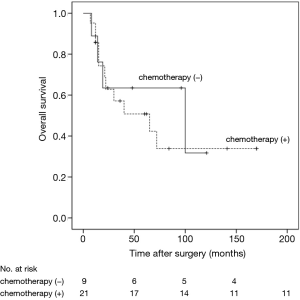
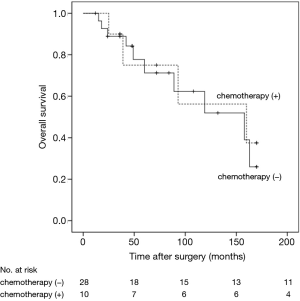
The 5- and 10-year survival rates for the 68 cases were 70.6% and 61.8%, respectively (Figure 5). In univariate analyses, factors associated with OS were age (HR =2.02, 95% CI: 0.95–4.28, P=0.024) and nodal status (HR =2.16, 95% CI: 1.03–4.54, P=0.046). Multivariate Cox regression analyses of OS of all 68 patients indicated that only nodal status (HR =2.20, 95% CI: 1.05–4.60, P=0.034) was significantly associated with survival (Table 1). For those patients with lymph-nodes involvement, no statistically significant difference was found in OS between those who received adjuvant chemotherapy treatment after resection and those who had operation alone (HR =1.23, 95% CI: 0.39–3.89, P=0.718) (Figure 6). In the measure of OS of N0 patients who had operation alone and those who received adjuvant chemotherapy, no significant difference was found between the two groups (HR =0.83, 95% CI: 0.26–2.70, P=0.761) (Figure 7).
Discussion
In our series, AC were more prevalent in men than in women with male to female ratio of 4.2:1, supported by many reports that revealed a male predominance for AC (3,4). In a large multicentric study, the mean age of AC was 56 years (5), which was also supported by the reports of El Jamal et al. and Filosso et al. (6,7). In our series, the mean age of patients with AC was 55 years in accordance with other studies.
In most of the published series, patients with AC had a higher incidence of centrally located tumors than peripheral (8-10), though there is some disagreement in the literature (3,11). In our data, 58.8% of tumors were localized peripherally while 41.2% of tumors were localized centrally. Centrally located tumors were more often symptomatic (8,12). In our study, 23 of the 27 (85.2%) centrally located AC were symptomatic, while 24 of the 41 (58.5%) peripherally located AC were symptomatic. The most common symptom was cough followed by hemoptysis and dyspnea. With the application of CT, more and more potentially asymptomatic peripheral AC will be detected.
Radical surgical resection remains the most effective treatment of AC. For peripheral tumors, lobectomy was considered to be a standard surgical treatment for AC (13). Because of high recurrence rates, limited resections such as wedge resection and segmentectomy were not recommended for AC, unless patients lacked sufficient pulmonary reserve (14). In our study, only one patient with peripheral tumor underwent wedge resection because of poor pulmonary function, and recurrence was observed 3 months after operation. For central tumors, reports had demonstrated that bronchoplastic surgery did not increase operative risk and local recurrence but also preserve lung parenchyma as much as possible (4,15). As a result, bronchoplastic surgery should be preferred whenever possible. However, in some cases there was no other way to avoid pneumonectomy, such as the inflammatory involvement of lung parenchyma or the location of tumor. In our data, only 2 patients (2.9%) underwent sleeve lobectomy, while 10 patients (14.7%) underwent pneumonectomy.
Ki-67 is a marker of cellular proliferation, which provides an objective measurement to quantify the proliferation index and is used in numerous cancers (16). Clay et al. (17) reported that Ki-67 index was independent prognostic factors for RFS in pulmonary carcinoid tumors. Grimaldi et al. (18) showed that there was a statistically significant correlation between the expression of Ki-67 and the long-term outcome for bronchopulmonary carcinoids. In our study, patients were classified according to Ki-67 levels in three subgroups (>0 and <5%, ≥5% and <10%, and ≥10%) and RFS was significantly worsened by increasing Ki-67 index (Ki-67 <5% vs. 5%≤ Ki-67 <10%, HR =11.26, 95% CI: 1.13–112.59, P=0.039; Ki-67 <5% vs. Ki-67 ≥10%, HR =31.54, 95% CI: 3.56–279.29, P=0.002).
Lymph-nodes involvement as a poor prognostic factor affecting long-term survival had been reported by several authors. Aydin et al. (9) showed that lymph-nodes involvement (especially N2) to be a bad prognostic factor for AC. Cardillo et al. (19) found a significant better survival for N0 patients compared with N1 and N2 patients (5-year survival 100%, 84.2% and 22.2%; P<0.001).
Our data supported these reports: RFS of N0 patients, N1patients and N2 patients was 84.2%, 73.3% and 33.3%, respectively. OS of N0 patients, N1 patients and N2 patients was 63.2%, 53.3% and 46.7%, respectively. Univariate and multivariate analyses both demonstrated that lymph-nodes involvement significantly worsened RFS and long-term survival. A multi-centre study of 247 atypical pulmonary carcinoids demonstrated that only lymph nodal involvement was a significantly adverse prognostic factor affecting survival in a multivariate analysis (P=0.008) (20). In this research, distant metastases occurred in 34.3% of patients with pathological lymph nodal involvement (pN+), which is higher than the recurrence rate in our data. Consistent with the above study, we hold the point that systematic mediastinal lymph node dissection was recommended for patients with AC which plays a key role as a prognostic factor for the management of AC and a close follow-up is strictly advised for patients with lymph nodal involvement.,
It is an accepted practice that patients with AC that has spread to lymph nodes receive adjuvant chemotherapy. However, a retrospective study of the National Cancer Data Base (NCDB) showed that the use of adjuvant chemotherapy postoperatively in patients with pathologically lymphnode-positive (pN+) and pathologically lymphnode-negative (pN−) disease conferred no survival advantage for patients with AC (21). Our findings corroborated the result: for those patients with lymph-nodes involvement, no statistically significant difference was found in RFS and OS between those who received adjuvant chemotherapy after resection and those who had operation alone (P=0.957 and P=0.718, respectively). For those patients without lymph-nodes involvement, no statistically significant difference was found in RFS and OS between those who received adjuvant chemotherapy after resection and those who had operation alone (P=0.698 and P=0.761, respectively).
This study is retrospective in nature, which makes it susceptible to selection bias of patients in the application of adjuvant chemotherapy. Our study is also limited by the small number, so the strength of the conclusions is limited in scope and generalizability.
Conclusions
In summary, long-term survival and recurrence are both unfavorably affected by lymph-nodes involvement in AC. Ki-67 level appears to be associated with recurrence. There was no significant difference in survival whether adjuvant chemotherapy was provided for patients with pN+ and pN- disease. Larger and randomized studies in the future should be conducted to determine proper chemotherapy use for these patients.
Acknowledgements
Funding: The study was funded by the grant 2016YFC0905400 from the National Key R&D Program of China.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the ethics committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (approval number: NCC201805013). The requirement of patients’ informed consent was waived owing to the retrospective nature of the study.
References
- Herde RF, Kokeny KE, Reddy CB, et al. Primary Pulmonary Carcinoid Tumor: A Long-term Single Institution Experience. Am J Clin Oncol 2018;41:24-9. [PubMed]
- Tsuta K, Raso MG, Kalhor N, et al. Histologic features of low and intermediate-grade neuroendocrine carcinoma (typical and atypical carcinoid tumors) of the lung. Lung Cancer 2011;71:34-41. [Crossref] [PubMed]
- Marty-Ané CH, Costes V, Pujol JL, et al. Carcinoid tumors of the lung: do atypical features require aggressive management? Ann Thorac Surg 1995;59:78-83. [Crossref] [PubMed]
- Rea F, Rizzardi G, Zuin A, et al. Outcome and surgical strategy in bronchial carcinoid tumors: single institution experience with 252 patients. Eur J Cardiothorac Surg 2007;31:186-91. [Crossref] [PubMed]
- García-Yuste M, Matilla JM, Alvarez-Gago T, et al. Prognostic factors in neuroendocrine lung tumors: a Spanish Multicenter Study. Spanish Multicenter Study of Neuroendocrine Tumors of the Lung of the Spanish Society of Pneumonology and Thoracic Surgery (EMETNE-SEPAR). Ann Thorac Surg 2000;70:258-63. [Crossref] [PubMed]
- El Jamal M, Nicholson AG, Goldstraw P. The feasibility of conservative resection for carcinoid tumours: is pneumonectomy ever necessary for uncomplicated cases? Eur J Cardiothorac Surg 2000;18:301-6. [Crossref] [PubMed]
- Filosso PL, Rena O, Donati G, et al. Bronchial carcinoid tumors: surgical management and long-term outcome. J Thorac Cardiovasc Surg 2002;123:303-9. [Crossref] [PubMed]
- Fink G, Krelbaum T, Yellin A, et al. Pulmonary carcinoid: presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest 2001;119:1647-51. [Crossref] [PubMed]
- Aydin E, Yazici U, Gulgosteren M, et al. Long-term outcomes and prognostic factors of patients with surgically treated pulmonary carcinoid: our institutional experience with 104 patients. Eur J Cardiothorac Surg 2011;39:549-54. [Crossref] [PubMed]
- Zhong CX, Yao F, Zhao H, et al. Long-term outcomes of surgical treatment for pulmonary carcinoid tumors: 20 years’ experience with 131 patients. Chin Med J (Engl) 2012;125:3022-6. [PubMed]
- Descovich P, Ansaloni L, Grazia M, et al. Bronchial carcinoids. Our experience with 35 cases. Minerva Chir 2000;55:113-9. [PubMed]
- Thomas R, Christopher DJ, Balamugesh T, et al. Clinicopathologic study of pulmonary carcinoid tumours—a retrospective analysis and review of literature. Respir Med 2008;102:1611-4. [Crossref] [PubMed]
- Ginsberg JR. Carcinoid tumors. In: Shields TW, LoCicero III J, Ponn RB. editors. General Thoracic Surgery. 5th ed. Vol 1. Philadelphia: Lippicott Williams & Wilkins, 1999: 1493-504.
- Mezzetti M, Raveglia F, Panigalli T, et al. Assessment of oucomes in typical and atypical carcinoids according to latest WHO classification. Ann Thorac Surg 2003;76:1838-42. [Crossref] [PubMed]
- Terzi A, Lonardoni A, Feil B, et al. Bronchoplastic procedures for central carcinoid tumors: clinical experience. Eur J Cardiothorac Surg 2004;26:1196-9. [Crossref] [PubMed]
- Skov BG, Holm B, Erreboe A, et al. ERCC1 and Ki67 in small cell lung carcinoma and other neuroendocrine tumours of the lung: distribution and impact on survival. J Thorac Oncol 2010;5:453-9. [Crossref] [PubMed]
- Clay V, Papaxoinis G, Sanderson B, et al. Evaluation of diagnostic and prognostic significance of Ki-67 index in pulmonary carcinoid tumours. Clin Transl Oncol 2017;19:579-586. [Crossref] [PubMed]
- Grimaldi F, Muser D, Beltrami CA, et al. Partitioning of bronchopulmonary carcinoids in two different prognostic categories by ki-67 score. Front Endocrinol (Lausanne) 2011;2:20. [Crossref] [PubMed]
- Cardillo G, Sera F, Di Martino M, et al. Bronchial carcinoid tumors: nodal status and long-term survival after resection. Ann Thorac Surg 2004;77:1781-5. [Crossref] [PubMed]
- Daddi N, Schiavon M, Filosso PL, et al. Prognostic factors in a multicentre study of 247 atypical pulmonary carcinoids. Eur J Cardiothorac Surg 2014;45:677-86. [Crossref] [PubMed]
- Anderson KL Jr, Mulvihill MS, Speicher PJ, et al. Adjuvant chemotherapy does not confer superior survival in patients with atypical carcinoid tumors. Ann Thorac Surg 2017;104:1221-30. [Crossref] [PubMed]