Flail chest stabilization with Nuss operation in presence of multiple myeloma
Introduction
Although skeletal involvement is common in multiple myeloma, flail chest with paradoxical thoracic wall movement and sternum instability is very rare. There have been only a few cases of flail chest complicating multiple myeloma. Fleegler et al. (1) reported a case of pathological flail chest complicating multiple myeloma. Abisheganaden et al. (2) described the use of bilevel positive airway pressure ventilatory support for pathological flail chest complicating multiple myeloma. However, there have has been no standard treatment method for this clinical condition. Surgical stabilization of flail chest is controversial. It has been shown that select patients benefit from this method. Several operative techniques using Kirshner wires, Judet’s struts, polypropylene mesh, and malleable metal plates have been developed; however, these methods are inadequate for patients with comorbid medical conditions. The Nuss operation described first in 1998 by Donald Nuss is a safe and minimally invasive one for pectus excavatum (3). We describe a male patient with multiple myeloma who presented with paradoxical movement of chest wall, chronic pain, and dyspnea on exertion as well as severe respiratory failure following blunt trauma and was successfully treated by the Nuss operation for internal stabilization.
Case report
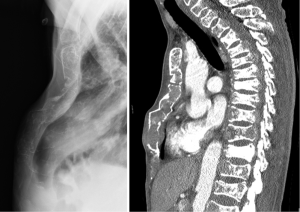
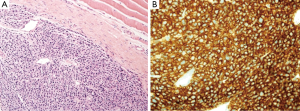
A 35-year-old male patient was referred from a regional hospital to our department for further management of chest pain, dyspnea, and oliguria. Two months ago, he had sustained mild blunt thoracic trauma at a traffic accident with the safety belt being fastened around the waist. At the regional hospital, the sternum fracture had been managed conservatively, and the compression fracture of the 11th thoracic vertebral body was treated by vertebraoplasty. The patient had been bedridden for approximately two months. At presentation, he had no underlying disease except sternum fracture and compression fracture of the 11th thoracic vertebral body. He had a white-collar job by occupation and did not have any specific family history, including the cancer. His blood pressure was 110/70 mmHg, pulse rate 82 beats/min, respiratory rate 16/min, and body temperature 36.7 °C. Physical examination revealed a 4-cm subcutaneous bulging mass in the left 10th rib and mild tenderness around the mass. Paradoxical movement of the right upper chest wall and sternum was observed, and crackles and inspiratory friction rubs were audible in the entire lung field. Complete blood counts revealed white blood cells, 14,800/µL; hemoglobin, 9.0 g/dL; platelets 130,000/µL; and erythrocyte sedimentation rate 42 mm/h. Blood chemical test results were as follows: albumin, 3.6 g/dL; blood urea nitrogen, 163.9 mg/dL; creatinine, 13.3 mg/dL; calcium, 11.4 mg/dL; C-reactive protein, 3.59 mg/dL; and uric acid, 15.3 mg/dL. Urine protein was 2+ (100 mg/dL) on urinalysis. Arterial blood gas analysis (ABGA) revealed pH, 7.39; PaO2, 77.6 mmHg; PaCO2, 34.0 mmHg; and oxygen saturation (SpO2), 95.5%. Plain chest radiographs, including the sternum lateral view and both rib oblique views, exhibited diffuse osteopenia, multiple rib fractures, sternum fracture and left the 10th rib destruction. Chest computed tomography (CT) revealed extensive osteoclastic lesions and bony destruction of multiple the sternum, spine ribs, as well as a 3×4-cm soft tissue mass in the left 10th rib (Figure 1). Further evaluation by whole body bone scintigraphy revealed multifocal increased or decreased uptakes in the whole axial and proximal appendicular skeletons, diffusely increased whole periarticular uptakes, and slightly increased uptakes in the sternum and ribs, especially in the mass of the left 10th rib with a bone to soft tissue ratio of 2.56:1. For the management of acute renal failure, hemodialysis was started immediately, and further workups were continued. Measurement of serum immunoglobulin (Ig) and immunoelectrophoresis revealed the following results: increased beta2-microglobulin level, 14.38 ug/mL (normal range, 1.0-3.0 ug/mL); decreased IgM, 12.0 mg/dL (normal range, 40-230 mg/dL); IgG, 388.0 mg/dL (normal range, 700-1,600 mg/dL); IgA, 15.0 mg/dL (normal range, 70-400 mg/dL), and IgD, <0.45 mg/dL (normal range, 0.77-13.21 mg/dL). Serum protein immunochemistry results were as follows: the serum decreased kappa-light chain, 87.1 mg/dL (normal range, 170-370 mg/dL) and increased lambda-light chain, 489.0 mg/dL (normal range, 90-210 mg/dL) (Table 1). Immunofixation electrophoresis of the serum and urine showed abnormal bands in the lambda lane. Under local anesthesia, incisional biopsy of the mass in the left 10th rib was performed, and it showed atypical plasma cells having centrally or eccentrically located ovoid nuclei and eosinophilic cytoplasm. It had been proved that this mass was consisted with a relatively well circumscribed tumor, and composed of monomoprhic plasmacytoid cells (Hematoxylin and Eosin, ×200). The tumor cells show diffuse and strong immunoreactivity against CD138 antibody (×400) (Figure 2). Immunohistochemical studies of the specimen showed positive lambda-chains and negative kappa-chains. This histologic finding indicates a plasma cell neoplasm suggestive of plasmacytoma of the rib. Histologic findings obtained from bone marrow biopsy were suggestive of plasma cell myeloma. Immunohistochimical staining of the specimen was positive for the cluster of differentiation (CD) 138 and for the lambda with monotypic lambda Ig expression, but negative for CD3, CD20, and kappa.
Full table
The patient was diagnosed with multiple myeloma with rib and sternum involvement based on laboratory test results and histological findings. His final diagnosis was Ig D-lambda-type multiple myeloma with Durie-Salmon stage IIIB and International staging system IIIB, so he was transferred to the Department of Hemato-oncology and started to receive chemotheraphy with the VAD regimen (vincristine + doxorubicin + dexamethasone). However, he complained of more aggravated thoracic wall pain, dyspnea, and paradoxical movement of the chest wall. ABGA results were still abnormal: pH, 7.24; PaO2, 62 mmHg; PaCO2, 46.0 mmHg; and SpO2, 85%. We considered further management of flail chest with paradoxical thoracic wall movement, including mechanical ventilation and surgical stabilization with an external fixator. Nonetheless, since less invasive but more effective management was required due to underlying multiple myeloma, we decided to perform the Nuss operation 107 days after blunt thoracic trauma. Under general anesthesia, the patient was placed in the supine position with both arms abducted. Two small incisions were made bilaterally in the center of flail segment on the mid-axillary line. After creation of subcutaneous tunnels to the hinge points, a specially designed transilluminated introducer was passed through the mediastinum under the depressed sternum instead of a pectus clamp. A 28-F chest tube was used as a guide to pull through the bent metal bar (13-inch pectus bar, MX-barTM, MedixAlign Tech, Seoul, Korea). By rotating the bar, the convexity of the bar lifted the depressed portion of the flail segment. Both ends of the bar and one hingepoint were fixed to adjacent ribs with wires (Surgical steel, EthiconTM, Inc. Somerville, NJ, USA).
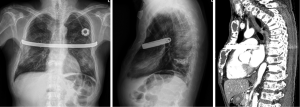
The patient’s postoperative course was uneventful, restored with thoracic stability (Figure 3). He was relieved from paradoxical movement of thoracic wall and pain, and ABGA results returned to normal. On the first postoperative day, the ABGA results without O2 supply was improved: pH, 7.39; PaO2, 98.8 mmHg; PaCO2, 34.2 mmHg; and SpO2, 99%. He was consulted to the Department of Rehabilitation Medicine for the further managements, including ambulation, and started ambulation with aids after bed side physical therapy on the 14th postoperative day. His Eastern Cooperative Oncology Group Performance Status Scale was changed from grade 4 to 2 on the 50th postoperative day after the third session of VAD chemotherapy. On the 97th postoperative day, he was discharged from the hospital and followed up at the Department of Hemato-oncology, without further dyspnea for two years.
Discussion
Medical or surgical stabilization of the segment of paradoxical chest wall movement is important for patients with flail chest. Currently, general acceptance of surgical stabilization has been gradually increased through improved surgical stabilization of flail chest with the development of variable rib fixation prostheses, e.g., anatomic rib plates, intramedullary splints, and absorbable nails (4,5). Several previous studies have demonstrated that surgical stabilization can significantly reduce the duration of mechanical ventilation, the incidence of pulmonary complications, the duration of ICU stays/hospitalization, and the incidence of chest deformities compared to internal pneumatic stabilization by using ventilator appliances (6). Nevertheless, surgical fixation is nearly impossible in patients with metastatic bone disease, such as multiple myeloma, which weakens the rib and sternum and in patients with compromised general medical conditions which induce high perioperative mortality and morbidity. To date, flail chest associated with multiple myeloma has been treated conservatively with mechanical ventilation or bilevel positive airway pressure ventilator support (2). Recently, several less invasive surgical fixation techniques, including percutaneous osteoplasty and intramedullary rib splints, have been proposed (7,8). Pacheco et al. (9) has reported the Nuss operation in a massive flail chest induced in a snowmobile accident and asserted that Nuss bars can be used to stabilize severe flail chest injuries, when chest reconstruction is necessary but fractured segment fixation is infeasible due to adjacent chest wall instability. In our case, since we were accustomed to the Nuss operation for the correction of pectus excavatum, we decided to perform the Nuss operation, a minimally invasive technique, because lateral thoracic structures were intact and flail segment was just the upper part of the anterior chest wall, including the upper half sternum and the second to fourth ribs.
Though not all patients with flail chest can be treated with the Nuss operation, especially in patients with the lack of intact lateral bony structures, the Nuss operation may be a useful for stabilizing of paradoxical movement of the chest wall, in select patients with the advantage of being minimally invasive. A case series would be required despite the rare occurance to determine if the Nuss operation can be a useful tool in stabilization of flail chest in these select patients.
Acknowledgements
This work was supported by Konkuk University.
Disclosure: The authors declare no conflict of interest.
References
- Fleegler B, Fogarty C, Owens G, et al. Pathologic flail chest complicating multiple myeloma. Arch Intern Med 1980;140:414-5. [PubMed]
- Abisheganaden J, Chee CB, Wang YT. Use of bilevel positive airway pressure ventilatory support for pathological flail chest complicating multiple myeloma. Eur Respir J 1998;12:238-9. [PubMed]
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg 1998;33:545-52. [PubMed]
- Fitzpatrick DC, Denard PJ, Phelan D, et al. Operative stabilization of flail chest injuries: review of literature and fixation options. Eur J Trauma Emerg Surg 2010;36:427-33. [PubMed]
- Bottlang M, Long WB, Phelan D, et al. Surgical stabilization of flail chest injuries with MatrixRIB implants: a prospective observational study. Injury 2013;44:232-8. [PubMed]
- Slobogean GP, MacPherson CA, Sun T, et al. Surgical fixation vs nonoperative management of flail chest: a meta-analysis. J Am Coll Surg 2013;216:302-11.e1.
- Zhou B, Wu CG, Li MH, et al. Percutaneous osteoplasty for painful sternal lesion from multiple myeloma. Skeletal Radiol 2009;38:281-5. [PubMed]
- Helzel I, Long W, Fitzpatrick D, et al. Evaluation of intramedullary rib splints for less-invasive stabilisation of rib fractures. Injury 2009;40:1104-10. [PubMed]
- Pacheco PE, Orem AR, Vegunta RK, et al. The novel use of Nuss bars for reconstruction of a massive flail chest. J Thorac Cardiovasc Surg 2009;138:1239-40. [PubMed]


