Changes in coagulation factor XII and its function during aortic arch surgery for acute aortic dissection—a prospective observational study
Introduction
Patients who suffer from acute aortic dissection (AAD) involving the ascending aorta (type A, according to the Stanford classification) require urgent surgical treatment to avoid sudden death and serious complications (1). However, the complex surgical methods and long operating time associated with this treatment can result in bleeding due to the coagulopathy that is associated with cardiopulmonary bypass (CPB) and moderate hypothermia circulation arrest (MHCA). Therefore, aortic surgery is associated with several complications, resulting in a worse prognosis compared with other cardiac surgeries (1). Many studies have focused on changes in the coagulation system during the perioperative period by monitoring key elements including platelets, thrombin and fibrinogen, and these studies have achieved definitive results (2,3). The extrinsic pathway protein factor VII has been proven to be an important clotting element, and its synthetic biological agent has also been applied in the clinic with tangible effects (4,5).
The intrinsic coagulation pathway, despite being an important part of the coagulation system, has not received enough attention with regard to this disease. The intrinsic coagulation pathway includes initiating factor XII, two intermediate factors, XI and IX, and other upstream stimulating factors such as prekallikrein (PK), bradykinin (BK) and high-molecular-weight kininogen (HMWK). In a signaling cascade, factor XI is stimulated by factor XII, which then activates factor IX and other coagulation pathway proteins (6,7). Our present study aims to describe changes in the intrinsic coagulation pathway during the perioperative period, to investigate whether the pathway is activated during aortic dissection surgery, and to identify a new target for the treatment of coagulopathy during the perioperative period that might reduce hemorrhage-related complications and required blood transfusions. To achieve this goal, we measured relative biomarkers of the coagulation system using enzyme-linked immunosorbent assays (ELISA) and standard laboratory tests.
Methods
Study population
We continuously recruited 152 patients who were clinically proven by CT scan to have acute Stanford type A AAD and who underwent emergency aortic arch surgery involving MHCA at our institution from January 2014 to September 2015. Patients who were pregnant or who had congenital or acquired coagulative disorders, liver disease, previous cardiac surgical history, stroke or myocardial infarction within two months before the surgery, those who were on dialysis or diagnosed with sepsis, who used oral anticoagulant or antiplatelet treatment within one week before the surgery, or those who had used human recombinant coagulation factor VII during the perioperative period were excluded. All aortic arch replacements with or without aortic valve replacement were eligible for inclusion in our study. With the inclusive and exclusive criteria above, 88 patients were finally enrolled in a consecutive basis.
Study design
In this single-center prospective study, we analyzed the standard laboratory test results and biomarkers of the intrinsic and extrinsic coagulation pathways in 88 patients who underwent aortic arch surgery. All surgical procedures were performed by a single surgeon (X.L.W.). The Ethics Committee at the Anzhen Hospital approved the study protocol (Institutional Review Board File 2014019), and consent was obtained from the patients or their relatives. The primary endpoint of this study was to evaluate the state of the intrinsic coagulation pathway at hospital admission, during the operation, and in the postoperative period among patients with AAD.
Surgical procedures
Standard anesthetic management was used with endotracheal intubation. The procedures were performed via a median sternotomy. The right axillary artery was used for arterial cannulation, and the right atrium was cannulated with a single atriocaval cannula. A left ventricular drain was inserted through the right upper pulmonary vein. After systemic heparinization (300 U/kg bodyweight and maintaining an activated clotting time longer than 480 seconds), CPB was established. During CPB, temperature-adjusted flow rates of 2.5 L/(min·m2) were used, and the mean arterial pressure was generally maintained between 50 and 70 mmHg. Our institutional preference was to perform total arch replacement using a tetrafurcate vascular graft combined with the implantation of a specific stented graft into the descending aorta. Right axillary arterial cannulation for antegrade cerebral perfusion [5–15 mL/(kg·min)] has been previously performed in our hospital. Our policy was to completely excise the primary tear based on the extent of disruption in each case. The arch was explored under MHCA at a nasopharyngeal temperature between 19 and 26 °C. After completing the distal anastomosis, CPB was reinstituted, and the patient was gradually rewarmed to a normal temperature after a 5-minute period of cold reperfusion for free radical washout. Proximal anastomosis was then performed.
Blood samples and coagulation assays
To examine the effects of aortic dissection and surgery on the activation of intrinsic coagulation factors, blood samples were obtained from all participants at five different timepoints as follows: anesthesia induction (T1), lowest nasopharyngeal temperature (T2), protamine reversal (T3), 4 hours after surgery (T4) and 24 hours after surgery (T5). The first 5 mL of blood drawn was discarded to eliminate the dilution effect of the saline. The sample was stored in a citrated blood collection tube. Blood was taken from the central venous catheter or peripheral vein and anticoagulated with sodium citrate to measure coagulation factors. Blood samples were centrifuged for 15 minutes at 3,500 rpm at 4 °C and frozen at −80 °C until they were assayed. White blood cells, hemoglobin, hematocrit, platelet count and fibrinogen concentration were assayed using an automated blood coagulation analyzer. Activation of the intrinsic and extrinsic coagulation pathways was assessed using the ELISA technique. Activation of the intrinsic coagulation pathway was evaluated by assessing the levels of coagulation factor XII (FXII; normal range: 55–95 U/mL), activated factor XII (FXIIa; normal range, 600–1200 U/L) and other intrinsic upstream stimulating factors, including PK (PK; normal range, 480–600 pg/mL), BK (BK; normal range, 4.0–6.0 ng/mL) and HMWK (HMWK; normal range, 8.5–12.0 µg/mL). The levels of factor VII (FVII; normal range, 3.37–5.50 IU/mL) and activated factor VII (FVIIa; normal range, 2.23–3.87 IU/mL) were assayed as markers of the extrinsic coagulation pathway. All ELISAs were performed twice, and the mean value was used for analysis.
Statistical analysis
Data were presented as the mean ± standard deviation (SD) for continuous data with a normal distribution, as the median (25th percentile, 75th percentile) for continuous data with a non-normal distribution, or as a number and percentage for categorical values. Differences between time points were analyzed using analysis of variance with repeated measures. Differences between two groups within the same timepoint were analyzed using the independent t-test, the Wilcoxon test and Pearson chi-square test for continuous data with a normal distribution, continuous data with a non-normal distribution and count data, respectively. Multivariable logistic regression was used to analyze independent risk factors. Statistical significance was defined as P<0.05 using two-tailed distributions. All statistical analyses were performed using computer software (SPSS 22.0, SPSS, Inc., Chicago, IL, USA).
Results
Patients grouping situation
Eighty-eight patients were divided into two groups according to whether underwent reoperation for coagulopathy after the emergency surgery. Of all the 12 patients who underwent reoperation for bleeding, 10 patients underwent reoperation because of coagulopathy, the other two patients underwent reoperation because of retrosternal active hemorrhage. The following criterion of reoperation was used: the 200 mL/h postoperative drainage lasting for 4–6 hours; 1,500 mL postoperative drainage in 12 hours; the postoperative pericardial tamponade, including unstable circulation fluctuated with breathing, poor peripheral circulation, high CVP and oliguria or anuria, and low systolic pressure and pulse pressure that could not be effectively managed by inotropic therapy.
Baseline characteristics
The majority of the patients with AAD had chest pain (95%) as the predominant preoperative symptom. Among all the patients, 75% were diagnosed with hypertension, and most of the patients had blood pressure that was difficult to control. All patients were admitted within 14 days of AAD onset with an average duration of 48 hours [25–75% interquartile range (IQR), 24–168 hours]. Routine preoperative assays demonstrated that patients with AAD had very high D-dimer levels and increased white blood cell counts. Other important baseline characteristics of the patients from the two groups are depicted in Table 1.
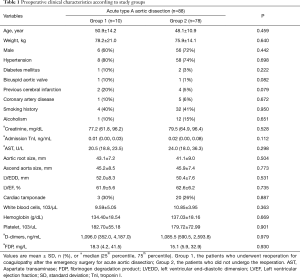
Full table
Details of the perioperative period
Overall, with the exception of two patients who underwent Wheat’s (ascending aorta and aortic valve replacement with a reserved autologous aortic sinus) or David (aortic root replacement with a reserved aortic valve) surgery, the vast majority of patients underwent composite graft or ascending replacement with total arch replacement using a tetrafurcate vascular graft combined with implantation of a specific stented graft into the descending aorta. As expected, patients with AAD required a longer duration of surgery (7.9±1.7 hours), longer duration of CPB [208 minutes (25–75% IQR, 107–456 minutes)] and longer aortic cross clamp time (131.1±31.5 minutes in Group 1 & 122.0±45.3 minutes in Group 2) (Table 2) compared with other cardiac surgeries. The average time of MHCA was 29.0±9.7 minutes in Group 1 & 27.7±10.1 minutes in Group 2 (Table 2), and all surgeries were performed by a single surgeon. Among all the patients who were included in our study, 9 patients died because of various complications (the in-hospital mortality was 10.3%), and the mean length of stay in the ICU was 6.9 days. Fourteen patients (15.9%) suffered from uncontrollable sepsis, 19 patients (21.6%) suffered respiratory failure of different degrees, 18 patients (20.5%) underwent postoperative dialysis, and postoperative cerebral infarction or hemorrhage was observed in 8 patients (9.1%). Furthermore, patients with AAD had high rates of postoperative bleeding and blood transfusions. Twelve patients (13.6%) required reoperation for bleeding, including ten patients (11.4%) who required reoperation for coagulopathy, and two patients (2.2%) who received reoperation because of retrosternal active hemorrhage. The intraoperative blood loss was 1,400 mL and the total postoperative drainage was 2,240 mL. The total dosage of packed red blood cells (PRBCs) was 900 mL during the perioperative period, and the total dosage of fresh frozen plasma (FFP) was 400 mL. The total dosage of platelet concentrate was (PC) 1.0 IU (1.0 IU PC approximately equals 250 mL). The total dosage of fibrinogen was 2.0 g, the total dosage of prothrombin complex was 800 IU, the total dosage of protamine was 500 mg, and the total dosage of heparin was 240 mg. Other detailed information of the two groups during surgery are described in Table 2.
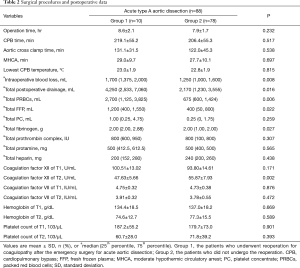
Full table
Standard laboratory tests
The results of standard laboratory tests are shown in Table 3. Preoperative routine assays demonstrated that white blood cells, hemoglobin, hematocrit, and platelet counts were significantly decreased compared with baseline values (P<0.01) during CPB. White blood cells recovered to a similar level compared with preoperative measurements within 24 hours after surgery. However, hemoglobin, hematocrit, and platelet counts remained at low levels for 24 hours after surgery (P<0.01). In this study, all patients exhibited hypofibrinogenemia, and their fibrinogen levels frequently decreased to <1.5 g/L during CPB. After hemostatic therapy, the fibrinogen level increased gradually and returned to baseline values within 24 hours after surgery.
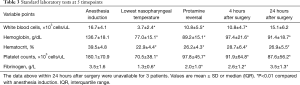
Full table
Coagulation tests
The intraoperative blood loss (P=0.008) and total postoperative drainage (P=0.016) between the two groups were significantly different, which was reasonable according to the grouping. The level of coagulation factor XII at T2 (P=0.002), the total dosage of fibrinogen (P=0.027), the total dosage of PRBCs (P=0.006) and the total dosage of FFP (P=0.022) during the perioperative period showed significant differences between the two groups (Table 2).
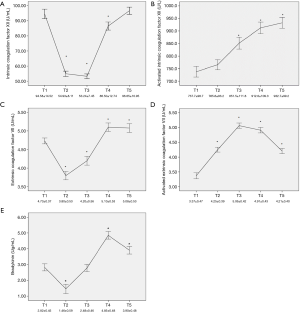
Factor XII, which is the core element of the intrinsic coagulation pathway, remained within a normal range before the operation. During MHCA, this factor dramatically dropped to a low level and gradually recovered to preoperative levels within 24 hours (P<0.001) (Figure 1A). Activated factor XII was also measured in our experiment, and it demonstrated a significantly gradual activating state (P<0.001) (Figure 1B). Meanwhile, factor VII, which is part of the extrinsic coagulation pathway, showed changes similar to factor XII, but it showed less of a decline during MHCA and recovered to a level above preoperative levels within four hours after surgery (P<0.001) (Figure 1C). Activated factor VII increased until the end of CPB, and within 24 hours after surgery, it showed a gradual decline (P<0.001) (Figure 1D). The preoperative level of BK was lower than the normal range and reached its lowest level during hypothermic circulatory arrest then gradually increased above preoperative levels and finally returned to the normal range 4 hours after surgery (P<0.001) (Figure 1E). Other stimulating factors, including PK, HMWK and histone-DNA-complex, did not show a significant change during surgery (P>0.05). Compared with factor VII, intrinsic coagulation factor XII demonstrated a higher proportion of decline during the operation (its proportion of decline from T1 to T2 was approximately 42%, while the proportion of decline from T1 to T2 for factor VII was approximately 20%, P<0.001). Moreover, factor XII underwent an intense reduction from T1 to T3, followed by a gradual increasing trend; however, it still did not return to preoperative levels at T4 (P=0.01). In contrast, although factor VII exhibited a similar trend from T1 to T3, it recovered to preoperative levels at T4 fairly quickly (P<0.001).
Multivariable logistic regression analysis on reoperation for coagulopathy
We analyzed the association between clinical variables and the risk of reoperation for coagulopathy in the above groups. The data indicated that the level of coagulation factor XII at T2 was associated with hospital reoperation for coagulopathy, with a statistically significant P value on the independent t-test (Table 2). In the multivariable logistic regression model, we considered these variables and other possible risk factors (including age, sex, weight, admission troponin I, hemoglobin at T1, platelet count at T1, CPB time, aortic cross clamp time, MHCA time, the level of coagulation factor XII at T1, the level of coagulation factor VII at T1 and the level of coagulation factor VII at T2) in the analysis. We found that the level of coagulation factor XII at T2 [odds ratio (OR): 1.342, 95% confidence interval (CI): 1.058–1.570; P=0.012] could independently predict the risk of reoperation for coagulopathy in patients with acute type A AAD (Table 4).
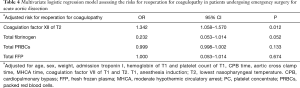
Full table
Discussion
All of the patients in our research were from Beijing Anzhen Hospital, which is one of the largest and most authoritative heart and aortic disease centers in China. Our study continuously and randomly collected the samples that fit the inclusion criteria and comprehensively described the total-field activation of key factors of the intrinsic and extrinsic coagulation pathways in patients within 24 hours after the surgical treatment of AAD.
Intrinsic coagulation pathway during the perioperative period
As reported above, factor XII expression was within the normal range before surgery and displayed marked activation and consumption throughout the perioperative period, whereas after surgery, it gradually recovered to preoperative levels. We also observed that activated coagulation factor XII continuously increased at each time point, which reflects the activation and consumption of factor XII in vivo.
The intrinsic coagulation pathway plays an important role in the formation of different types of coagulants. According to the coagulation cascade theory, coagulation factor XII, which is the initiation factor of the intrinsic pathway, can be processed into XIIa and can trigger downstream factors such as factor XI and IX via a signaling cascade; then, with the catalysis of activated factor X, prothrombin can transform to its activated form, thrombin. Many previous reports have stated that coagulants, such as thrombin, fibrinogen and plasminogen, undergo dramatic changes during total-arch replacement surgery in AAD patients (3). In the present research, our results regarding coagulation factor XII were comparable to those previously reported experiments, which might demonstrate a causal relationship between factor XII and coagulopathy in AAD. Among the intrinsic upstream stimulating factors, BK is the only factor that significantly synchronously changes with factor XII. It can be speculated that BK has a strong signaling link with factor XII, and the mechanism behind this phenomenon is worthy of further study.
Intrinsic coagulation pathway and extrinsic coagulation pathway
Our research showed that factor XII is more markedly affected by AAD surgery, especially during the process of MHCA. Moreover, decreased levels of factor XII during MHCA, but not during the perioperative period, is an independent risk factor of reoperation for coagulopathy. We speculate that factor XII might have a more important role in promoting thrombosis during the perioperative period for acute type A aortic dissection patients, and dynamic monitoring and timely compensation for factor XII during surgery might decrease bleeding-related complications and reduce the need for blood transfusions. Since recombinant human factor VII could have been in use and received effective clinical results, factor XII and other relevant intrinsic elements should be a focus during follow-up tests and in animal experiments, which might aid the development of hemostatic adjustment strategies during AAD.
The extrinsic coagulation pathway, in comparison with the intrinsic pathway, is activated by TF, which forms the VIIa-TF complex. In previous research, we found that the activation and consumption of thrombin in surgeries with CPB and HCA might be the most important cause of perioperative bleeding (3). After a more detailed analysis of our present research, we observed that XIIa had a similar linear upward trend as thrombin during the perioperative period, as determined in our previous research (6). In contrast, VII presented a similar linear trend from the onset of surgery to the hypothermia period. However, from 4 to 24 hours after the operation, the level of VIIa significantly decreased. There are three potential reasons for this phenomenon. First, as the main factor in the process of thrombosis, the consumption of factor VII before surgery can lead to postoperative insufficiency and a decrease in activated factor VII. Second, extrinsic factor VII is considered to play an important role in the early stages of thrombosis, whereas intrinsic factor XII is more likely to function in the maintenance of thrombus stability in the false lumen and in preventing postoperative bleeding. Third, the influence on the half-life and liver synthesis of FXII compared with FVII is also important. The half-life of FVII is rather fast, and its fast consumption leads to a quick recovery; FXII has a relatively slower half-life that will require a slower recovery. At this point, we reached a similar conclusion as previous studies in thrombosis research (8) and propose the implementation of a more profound study of the clinical applications for coagulation factors XII and VII.
Intrinsic coagulation factors, platelets and the inflammatory reaction
The intrinsic coagulation pathway is initiated by the exposure of collagen after endothelial cell damage (9); the aggregation, adhesion and activation of platelets and the secretion of various cytokines from platelets are also caused by the exposed collagen. In addition, the results from previous research suggest that platelets exhibited a similar changing trend as clotting factor XII (2). In fact, some studies have suggested that there exists a causal relationship between the blood coagulation system and platelet activation (10,11). Because these two factors play important roles in aortic dissection, further research on the interaction between the platelet system and the intrinsic clotting pathway might be valuable for pathogenesis research and for the clinical treatment of aortic dissection during follow-up studies.
Many reports have shown that inflammatory reactions might occur and persist for some time during the perioperative period (12,13); clinical and animal experiments have shown the relationship between inflammation and MHCA (14,15). As we observed in the present study, the white blood cell count and C-reactive protein increased to high levels within 24 hours after surgery (3). In addition to postoperative infections, dramatic changes in the coagulation system lead to the activation of inflammatory factors and might be one of the major reasons for inflammatory reactions. For example, as an important element of the anticoagulation system, antithrombin III (AT III) has a powerful inhibitory effect on thrombin and also has anti-inflammatory properties (16); lower AT III levels identify AAD patients who are at an increased risk for early mortality and adverse outcomes (17). Meanwhile, many clotting factors, such as factors XII and XI, and stimulating factors, such as histone-DNA-complex, kallikrein, PK and HMWK in the intrinsic clotting pathway, have proven to have powerful effects on inflammatory responses (18). Based on these theories, we believe that the relationship between the intrinsic coagulation pathway and the postoperative inflammatory reaction in aortic dissection patients should be considered for an in-depth study.
Conclusions
Factor XII is more highly influenced by AAD surgery and requires a longer period of time to recover to preoperative levels compared with factor VII, and the level of factor XII during hypothermia circulation arrest might be an independent risk factor for reoperation for coagulopathy. Therefore, the supplementation of coagulation factor XII and its upstream stimulating factors might be a promising therapeutic modality in the future.
Limitations
The major limitation was that the number of patients was insufficient, which affected the precision of some analyses, especially the number of reoperations for coagulopathy group. Therefore, it is advisable that the sample size should be expanded in future studies. Another limitation of our study was that we had no technical approach to accurately evaluate the degree of thrombosis in the false lumen, which might affect the coagulation system.
Acknowledgements
We acknowledge Ji Che and Jing Liu (Beijing Institute of Heart Lung and Blood Vessel Diseases and Beijing Anzhen Hospital, Capital Medical University, China) for reviewing the manuscript during its development.
Funding: This study was financially supported by grants from the National Key R & D Program of China (2017YFC1308000), the National Science Foundation of China (81770466 and 81600362), the General Program of Science and Technology Development Project of Beijing Municipal Education Commission of China No. KM201610025019, and the Basic Science Cooperation Projects of Capital of Medical University (No. 17JL71).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Ethics Committee at the Anzhen Hospital approved the study protocol (Institutional Review Board File 2014019), and consent was obtained from the patients or their relatives.
References
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons,and Society for Vascular Medicine. J Am Coll Cardiol 2010;55:e27-e129. Erratum in: J Am Coll Cardiol 2013;62:1039-40. [Crossref] [PubMed]
- Guan XL, Wang XL, Liu YY, et al. Changes in the Hemostatic System of Patients With Acute Aortic Dissection Undergoing Aortic Arch Surgery. Ann Thorac Surg 2016;101:945-51. [Crossref] [PubMed]
- Guan X, Li J, Gong M, et al. The hemostatic disturbance in patients with acute aortic dissection: A prospective observational study. Medicine (Baltimore) 2016;95. [Crossref] [PubMed]
- Lehr EJ, Alford TJ, Wang SH. Recombinant activated factor VII for postoperative hemorrhage following repair of acute type A aortic dissection. Heart Surg Forum 2010;13:E275-9. [Crossref] [PubMed]
- Grubitzsch H, Vargas-Hein O, Von Heymann C, et al. Recombinant activated factor VII for treatment of refractory hemorrhage after surgery for acute aortic dissection. J Cardiovasc Surg (Torino) 2009;50:531-4. [PubMed]
- van Montfoort ML, Meijers JC. Recent insights into the role of the contact pathway in thrombo-inflammatory disorders. Hematology Am Soc Hematol Educ Program 2014;2014:60-5. [Crossref] [PubMed]
- Schmaier AH. Physiologic activities of the contact activation system. Thromb Res 2014;133 Suppl 1:S41-4. [Crossref] [PubMed]
- Kuijpers MJ, van der Meijden PE, Feijge MA, et al. Factor XII regulates the pathological process of thrombus formation on ruptured plaques. Arterioscler Thromb Vasc Biol 2014;34:1674-80. [Crossref] [PubMed]
- Koenig JM, Chahine A, Ratnoff OD. Inhibition of the activation of Hageman factor (factor XII) by soluble human placental collagens types III, IV, and V. J Lab Clin Med 1991;117:523-7. [PubMed]
- Heemskerk JW, Mattheij NJ, Cosemans JM. Platelet-based coagulation: different populations, different functions. J Thromb Haemost 2013;11:2-16. [Crossref] [PubMed]
- Cheng Q, Tucker EI, Pine MS, et al. A role for factor XIIa-mediated factor XI activation in thrombus formation in vivo. Blood 2010;116:3981-9. [Crossref] [PubMed]
- Schillinger M, Domanovits H, Bayegan K, et al. C-reactive protein and mortality in patients with acute aortic disease. Intensive Care Med 2002;28:740-5. [Crossref] [PubMed]
- Koster A, Fischer T, Praus M, et al. Hemostatic activation and inflammatory response during cardiopulmonary bypass: impact of heparin management. Anesthesiology 2002;97:837-41. [Crossref] [PubMed]
- Kirklin JK, Westaby S, Blackstone EH, et al. Complement and the damaging effects of cardiopulmonary bypass. J Thorac Cardiovasc Surg 1983;86:845-57. [PubMed]
- Engels M, Bilgic E, Pinto A, et al. A cardiopulmonary bypass with deep hypothermic circulatory arrest rat model for the investigation of the systemic inflammation response and induced organ damage. J Inflamm (Lond) 2014;11:26. [Crossref] [PubMed]
- Warren BL, Eid A, Singer P, et al. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA 2001;286:1869-78. Erratum in: JAMA 2002;287:192. [Crossref] [PubMed]
- Sodeck GH, Schillinger M, Ehrlich MP, et al. Preoperative antithrombin III activity predicts outcome after surgical repair of acute type A aortic dissection. Atherosclerosis 2006;186:107-12. [Crossref] [PubMed]
- Barros NM, Tersariol IL, Oliva ML, et al. High molecular weight kininogen as substrate for cathepsin B. Biol Chem 2004;385:551-5. [Crossref] [PubMed]


