Effect of viral upper respiratory tract infection on cough reflex sensitivity
Introduction
Cough is among the most common reasons for which patients worldwide seek medical attention (1). Most cases of acute and subacute cough are due to acute viral upper respiratory tract infections (URI), otherwise known as the common cold. Given the sheer enormity of the problem of common cold worldwide, the medical and economic implications of cough due to this condition are of tremendous significance. Not only does acute cough due to URI result in tremendous financial expenditure, but the available treatments for acute cough are limited by lack of efficacy or, as in the case of opiates, for example, intolerable side effects at antitussive doses (2,3).
Measurement of cough reflex sensitivity
Measurement of cough reflex sensitivity has allowed insight into the effects of viral URI on human cough reflex sensitivity. Among the various provocative agents used for induction of cough in the laboratory, capsaicin, the pungent extract of red chili peppers, has gained favor as the tussive agent of choice, given its ability to induce cough in a safe (4), dose-dependent and reproducible manner (5,6). The typical end points of capsaicin inhalation challenge are C2 and C5, the concentrations of capsaicin inducing two or more, and five or more coughs, respectively. Figure 1 illustrates a typical dose-response curve generated during a capsaicin challenge study.
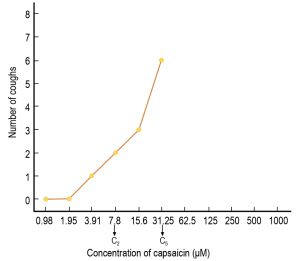
Effect of viral URI on cough reflex sensitivity
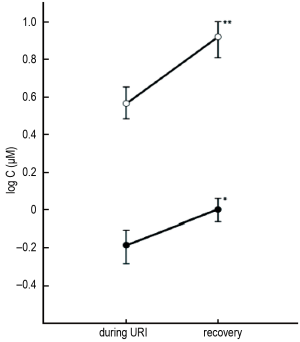
Employing capsaicin inhalation challenge to measure cough reflex sensitivity, O’Connell and colleagues initially demonstrated that cough reflex sensitivity is transiently enhanced in otherwise healthy subjects during acute viral URI, compared with the post-recovery state (7). These findings were subsequently confirmed in a similar population (8), as shown in Figure 2, with C5 significantly decreased (cough reflex sensitivity enhanced) during URI compared with repeated measurement after recovery (4-8 weeks post-URI).
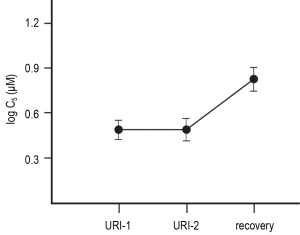
A recent study was the first to perform multiple cough reflex sensitivity measurements during the acute phase of viral URI in otherwise healthy subjects (9). Capsaicin cough challenge, performed twice during the first eight days of acute URI, demonstrated stability of cough reflex sensitivity during acute URI, with subsequent diminution (increased C5) post-recovery (Figure 3). This observation is of significance to future investigators evaluating the effect of a pharmacological intervention on acute cough, as changes in cough reflex sensitivity can be assumed to reflect the effect of the study drug, rather than significant, naturally occurring fluctuations in cough reflex sensitivity occurring during the early stages of acute URI.
Relatively few studies have evaluated the effect of a drug on cough reflex sensitivity during URI. The widely used expectorant, guaifenesin, has been shown in two clinical trials to inhibit cough reflex sensitivity in subjects with acute viral URI, but not in healthy volunteers (10,11). Similarly, the anticholinergic bronchodilator, tiotropium, has been shown to suppress cough reflex sensitivity in otherwise healthy nonsmokers with acute URI, but not in healthy controls (12). Interestingly, the antitussive effect of tiotropium occurred without demonstrable bronchodilation, thus highlighting the concept that tiotropium may have pharmacological effects beyond that of reversal of pathological airway obstruction (13). Notably, both of these agents inhibited capsaicin sensitivity in subjects with URI, whose cough reflex sensitivity was transiently enhanced, whereas the drugs had no effect in healthy volunteers, whose cough reflex was at its baseline. These observations raise the concept that subjects with pathologically enhanced cough reflex sensitivity, rather than healthy volunteers, comprise the optimal study population for clinical trials evaluating potential antitussive agents (14).
Mechanisms of enhanced cough reflex sensitivity during viral URI
Numerous mechanisms have been proposed to explain the transient cough and enhancement of cough reflex sensitivity associated with acute viral URI (15). Direct effects of the viral infection on airway epithelium include inflammation and cytokine release. Other airway effects of URI include increase in neurotransmitter levels, such as Substance P; reduced activity of neutral endopeptidases; increased neural receptor levels (NK-1); and, transient modulation of airway neural activity. Increased leukotriene production and mucus hypersecretion are likely additional contributors to cough induction. Viral infections induce bronchoconstriction and airway hyperresponsiveness through their influence on cholinergic pathways, but the significance of these effects on cough and cough reflex sensitivity remains to be elucidated (Table 1).

Full table
It is of interest, and likely quite significant, that many patients presenting with chronic cough relate the onset of their longstanding condition to an episode of URI. The newly emerging concept of the Cough Hypersensitivity Syndrome (16-19) suggests that an underlying hypersensitivity of the cough reflex potentiates the effect of an exogenous stimulus such as acute viral URI, resulting in refractory, chronic cough in a particular subgroup of individuals whereas the same stimulus causes a merely transient cough in the great majority of the population.
Effect of viral URI on the urge-to-cough (UTC) sensation
Recently, increasing interest has focused on the sensation of irritation that precedes the motor act of coughing; this phenomenon has been termed the UTC (20-23). Studies employing functional magnetic resonance imaging in subjects administered inhaled capsaicin have demonstrated that the UTC sensation is associated with activations in a variety of cortical brain regions (24,25). As is the case with the motor cough reflex, acute viral URI has been demonstrated to induce a transient enhancement of the UTC sensation (8). As shown in Figure 2, UTC, as measured by Cu, the lowest concentration of capsaicin inducing the UTC sensation without an associated motor cough (23), is significantly enhanced during URI compared to the post-recovery state.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Hsiao CJ, Cherry DK, Beatty PC, et al. National Ambulatory Medical Care Survey: 2007 summary. Natl Health Stat Report 2010;1-32. [PubMed]
- Dicpinigaitis PV. Cough: an unmet clinical need. Br J Pharmacol 2011;163:116-24. [PubMed]
- Dicpinigaitis PV, Colice GL, Goolsby MJ, et al. Acute cough: a diagnostic and therapeutic challenge. Cough 2009;5:11. [PubMed]
- Dicpinigaitis PV, Alva RV. Safety of capsaicin cough challenge testing. Chest 2005;128:196-202. [PubMed]
- Dicpinigaitis PV. Short- and long-term reproducibility of capsaicin cough challenge testing. Pulm Pharmacol Ther 2003;16:61-5. [PubMed]
- Morice AH, Fontana GA, Belvisi MG, et al. ERS guidelines on the assessment of cough. Eur Respir J 2007;29:1256-76. [PubMed]
- O’Connell F, Thomas VE, Studham JM, et al. Capsaicin cough sensitivity increases during upper respiratory infection. Respir Med 1996;90:279-86. [PubMed]
- Dicpinigaitis PV, Bhat R, Rhoton WA, et al. Effect of viral upper respiratory tract infection on the urge-to-cough sensation. Respir Med 2011;105:615-8. [PubMed]
- Dicpinigaitis PV, Tibb A, Hull D, et al. Cough reflex sensitivity during acute viral upper respiratory tract infection Eur Respir J 2011;38:626S.
- Dicpinigaitis PV, Gayle YE. Effect of guaifenesin on cough reflex sensitivity. Chest 2003;124:2178-81. [PubMed]
- Dicpinigaitis PV, Gayle YE, Solomon G, et al. Inhibition of cough-reflex sensitivity by benzonatate and guaifenesin in acute viral cough. Respir Med 2009;103:902-6. [PubMed]
- Dicpinigaitis PV, Spinner L, Santhyadka G, et al. Effect of tiotropium on cough reflex sensitivity in acute viral cough. Lung 2008;186:369-74. [PubMed]
- Bateman ED, Rennard S, Barnes PJ, et al. Alternative mechanisms for tiotropium. Pulm Pharmacol Ther 2009;22:533-42. [PubMed]
- Dicpinigaitis PV. Review: effect of drugs on human cough reflex sensitivity to inhaled capsaicin. Cough 2012;8:10. [PubMed]
- Footitt J, Johnston SL. Cough and viruses in airways disease: mechanisms. Pulm Pharmacol Ther 2009;22:108-13. [PubMed]
- Morice AH. The cough hypersensitivity syndrome: a novel paradigm for understanding cough. Lung 2010;188 Suppl 1:S87-90. [PubMed]
- Chung KF. Chronic ‘cough hypersensitivity syndrome’: a more precise label for chronic cough. Pulm Pharmacol Ther 2011;24:267-71. [PubMed]
- Morice AH, Faruqi S, Wright CE, et al. Cough hypersensitivity syndrome: a distinct clinical entity. Lung 2011;189:73-9. [PubMed]
- Morice AH, McGarvey LP, Dicpinigaitis PV. Cough hypersensitivity syndrome is an important clinical concept: a pro/con debate. Lung 2012;190:3-9. [PubMed]
- Davenport PW, Sapienza CM, Bolser DC. Psychophysical assessment of the urge-to-cough. Eur Respir Rev 2002;12:249-53.
- Davenport PW. Urge-to-cough: what can it teach us about cough? Lung 2008;186 Suppl 1:S107-11. [PubMed]
- Kanezaki M, Ebihara S, Nikkuni E, et al. Perception of urge-to-cough and dyspnea in healthy smokers with decreased cough reflex sensitivity. Cough 2010;6:1. [PubMed]
- Dicpinigaitis PV, Rhoton WA, Bhat R, et al. Investigation of the urge-to-cough sensation in healthy volunteers. Respirology 2012;17:337-41. [PubMed]
- Mazzone SB, McLennan L, McGovern AE, et al. Representation of capsaicin-evoked urge-to-cough in the human brain using functional magnetic resonance imaging. Am J Respir Crit Care Med 2007;176:327-32. [PubMed]
- Leech J, Mazzone SB, Farrell MJ. Brain Activity Associated with Placebo Suppression of the Urge-to-Cough in Humans. Am J Respir Crit Care Med 2013;188:1069-75. [PubMed]


