Smoking resumption after heart or lung transplantation: a systematic review and suggestions for screening and management
Introduction
According to projections of the World Health Organization (WHO), an estimated 1.1 billion people will smoke tobacco by 2025 (1). Tobacco smoking increases the risk for cardiovascular disease, stroke, peripheral vascular and lung disease, bacterial or viral infections and cancer (2,3). Despite a considerable rise in awareness and the implementation of smoking prevention strategies, combustible tobacco continues to be the major risk for preventable disease and death in the developed world, killing half of all long-term users (2,4).
Thoracic organ transplant (TOT) including heart transplantation (TPL), lung TPL or combined heart/lung TPL is an established treatment option for several non-malignant end-stage heart and lung diseases such as ischemic and non-ischemic cardiomyopathy, valvular cardiomyopathy, congenital heart disease, chronic obstructive pulmonary disease (COPD), interstitial lung disease (ILD), cystic fibrosis (CF) and pulmonary arterial hypertension (PAH) (5).
This review does not address other solid organ recipients (e.g., liver, kidney, pancreas), due to different TPL-waiting list requirements accepting current smokers.
Owing to the current shortage of donor organs, improving allograft outcomes is a pivotal aspect of ongoing clinical TPL research (6,7). Depending on the national organ donation organizations and TPL centers, patients are normally required to be smoke-free for a minimum of 6 months before being placed on the TPL-waiting list for thoracic organs TPL (8-10). Because smoking is an addictive disorder, relapse may occur post-transplant despite being smoke-free prior to TPL. Previous studies have demonstrated higher incidences of allograft dysfunction, development of de novo malignancies and reduced overall survival in smoking organ recipients (11-17).
To date, no international guidelines for systematic screening of post-transplant smoking recurrence exists. Therefore, it remains at the discretion of the individual TPL centers to systematically screen, question or rely on chance to detect smoking resumption after TPL. Despite considerable improvements in smoking cessation strategies including non-pharmacological (i.e., cognitive behavioral therapy, motivational interviewing) and pharmacological therapies (i.e., nicotine replacement therapy (NRT), partial nicotine agonists (varenicline, cytisine) and non-nicotine-based drugs (bupropion), its efficacy and safety transplant patients has rarely been investigated (18-22).
This review will examine the prevalence and outcome of smoking resumption in thoracic transplant recipients and how pre-transplant or post-transplant variables are associated with smoking reuptake. Such predictors, once identified, might help health care professionals and patients prevent smoking resumption after TOT in future. The importance of systematic screening of smoking resumption after TPL is discussed and tobacco cessation strategies in the context of transplant patients are reviewed.
Sources and selection criteria
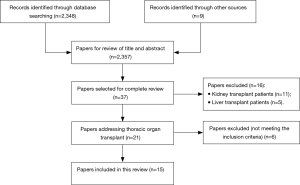
We searched the electronic databases Medline, Embase, Web of Science, Cochrane Library and Google scholar from database inception until March 5, 2017. Medical subject headings (MeSH) terms included “smoking” or “smoking resumption” or “smoking recurrence” or “smoking relapse” and “heart transplant” or “lung transplant”. Supplemental search was performed using keyword search for “smoking after transplant”. Eligible studies had to meet the following inclusion criteria; (I) characterize smoking resumption after TPL; (II) provide data on heart or lung transplant or combined heart/lung transplant recipients; (III) be reported in English with full text available. No other restrictions were applied. Furthermore, we scanned the reference sections of the eligible articles for additional literature and selected other relevant articles for further review by author consensus. Evidence came from a variety of study types, including case reports, randomized trials, systematic reviews and meta-analysis. Abstracts of meetings and unpublished results were not considered for this review. A total of 2,356 articles were screened. Thirty-seven articles were selected for complete review based on title and abstract, 15 of these were included in this review (Figure 1). Owing to the paucity of literature on this topic, no studies fulfilling the inclusion criteria were excluded because of being rated poor quality, despite being rated quality during data reviewing.
Systematic screening of smoking resumption after thoracic organ TPL
Different diagnostic methods have been suggested to identify nicotine consumption including self-report, semi quantitative and quantitative urine cotinine levels, serum cotinine levels, exhaled carbon monoxide (CO) or carboxyhemoglobin (COHb). CO can be measured in expired air or in the blood, COHb only in the blood using spectrophotometry. Both levels are highly correlated (23,24). CO has limited sensitivity in detection of light smoking because CO levels from smoking are low and can be influenced by environmental sources (i.e., air pollution, open fires) (25). Nicotine itself can be directly measured in the urine, blood or saliva and is in the absence of NRT highly specific, but not a feasible method due to its short half-life (18). Cotinine, a metabolite of nicotine, can be measured in the plasma and urine. Using a cutoff value of 15 and 50 ng/mL, respectively has the highest specificity (99%) and sensitivity (96%) for tobacco use (23,26).
At routine follow-up visits, however, smoking is mostly assessed only by questionnaire. In previous studies, self-report has shown a limited sensitivity of 25–85% for smoking resumption (27,28). Despite its high sensitivity and specificity, quantitative cotinine measurements are seldom used in routine patient encounters. Given the low prevalence of smoking resumption in a TPL cohort, systematic screening should only rely on quantitative cotinine measurements at routine follow-ups to identify smokers and thus identify patients for appropriate treatment.
Prevalence and consequences of smoking resumption
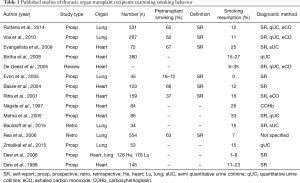
There are only few data available concerning the prevalence of smoking resumption in TOT recipients (Table 1). Rates of tobacco resumption range between 6–35% in heart and 0% to 15% in lung recipients (29-32). Of note, diagnostic assessments vary strongly in the considered studies (i.e., self-report, semi- and quantitative cotinine measurements, exhaled CO or COHb) (Table 1).
Full table
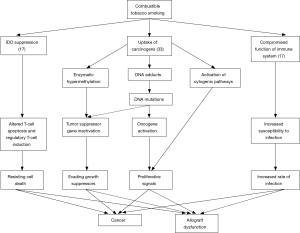
In mouse models, immunologic pathways potentially linking tobacco smoking to organ rejection have been identified. Messenger-RNA (mRNA) and protein expression of indoleamine 2,3-dioxygenase (IDO) is suppressed by tobacco smoking (17). IDO is expressed by antigen-presenting cells and metabolizes tryptophan to serotonin and kynurenine, both of which are involved in T-cell apoptosis and induction of regulatory T cells, pivotal aspects in allograft regulation and survival (17). Combustible tobacco contains over 60 well-established carcinogens, including polycyclic aromatic hydrocarbons, aromatic amines, aldehydes, N-nitrosamines, volatile organic compounds (VOCs) and metals (33). Through metabolic activation, generally catalyzed by CYP-450, carcinogens can covalently bind to DNA and form DNA-adducts leading to miscoding during replication (4). Other pathways (e.g., activation of cytogenetic pathways or enzymatic hypermethylation) have been identified as drivers in smoking related carcinogenesis (33). Furthermore, the carcinogenic effects of tobacco combustion appear to have synergistic effects with immunosuppressive therapy (33). While an increasing number of studies have shown the carcinogenic adverse effects of smoking in liver transplant recipients, clinical trials regarding smoking resumption in heart or lung recipients, however, have been controversial (Table 2) (34-36). Only three prospective studies involving a total number of 795 patients have shown significantly higher rates of malignancy, but only Nägele et al. have described a reduced overall survival among patients with post-transplant nicotine resumption (27,37,38). These findings could be biased by the fact that patients with allograft dysfunction may be less prone to restart smoking than patients without such complications (Figure 2).
Full table
Risk factors associated with post-transplant smoking resumption
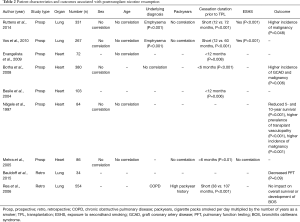
Prospective and retrospective studies of TOT patients examining risk factors for tobacco resumption are listed in Table 2. Interestingly, gender and age were not found to correlate with smoking resumption in any of the studies (27,28,31,37-42). Underlying diagnosis was found to be an independent risk factor for smoking resumption (27,37,39,42). This concurs with the implication of tobacco consumption in the pathogenesis of various pulmonary diseases, typical examples being emphysema and COPD. Regarding the smoking behavior before TPL, a short abstinence period before TPL was found to be an independent risk factor for resumption in all studies addressing this issue (27,28,37,39-42). Of interest, the number of packyears, a well-known surrogate for the degree of tobacco exposure and correlated risk of disease, was not shown to be a risk factor for resumption in 3 of 4 studies (27,39,41,42). Exposure to secondhand smoking (ESHS) was found to be a risk factor in two studies (27,39,41). Very little is known about pretransplant psychological testing of the risk for tobacco use resumption. In 2004, Basile et al. assessed patients before heart TPL using the Minnesota Multiphasic Personality Inventory (MMPI) (40,43). MMPI is a standardized psychometric test used to evaluate and describe personality and psychopathology. The authors concluded that patients who ceased smoking within one year before being included in the waiting list in combination with a lower degree of self-control and difficult adaptation scales are at risk for tobacco resumption (Table 2).
Smoking cessation interventions in transplant recipients
In recent years, considerable improvements in non-pharmacological and pharmacological interventions for smoking cessation have been made (44). Studies on interventions for TOT are scarce. A large meta-analysis by Cahill et al. involving a total of 267 studies (from non-transplant settings) and over 100,000 patients analyzed the treatment effects of the most relevant and widely used pharmacotherapies for smoking cessation (45).Varenicline has shown the highest probability of biochemically validated abstinence at 6 and 12 months [odds ratio (OR) 2.88, 95% confidence interval (CI): 2.40–3.47], followed by NRT (OR 1.84, 95% CI: 1.71–1.99) and bupropion (OR 1.82, 95% CI: 1.60–2.06) (45).
Counseling strategies alone, including motivational interviewing and cognitive behavioral therapy, have shown limited efficacy in smoking cessation (brief advice to quit: OR 1.66, 95% CI: 1.42–1.94; telephone counseling: OR 1.56, 95% CI: 1.38–1.77 and a combination of pharmacotherapies and counseling: OR 1.83, 95% CI: 1.68–1.98) (46-48).
None of the reviewed studies suggested or described smoking cessation strategies, safety, tolerance and effectiveness in TOT patients. This lack of information concerning smoking cessation interventions in this population draws attention to a field that needs to be investigated in future clinical trials.
Nicotine acts on nicotinic cholinergic receptors in the mesolimbic system releasing dopamine and serotonin, which sustains addiction (21). Nicotine is metabolized primarily by cytochrome P-2A6 (CYP2A6) (18). Pharmacologic studies have observed a slight induction of CYP2A6 by commonly used immunosuppressive drugs (i.e., cyclosporine A, tacrolimus, mycophenolate mofetil). However, a clinically relevant increased nicotine metabolism or withdrawal symptoms remains under question (49). NRT therapy is generally considered to be safe and can be used in transplant recipients as in the general population (50).
Varenicline, a nicotinic acetylcholine receptor partial agonist, is the most widely used and most efficient smoking cessation drug available (51). Because of its reported side effect profile, it should be prescribed in patients with a history of neuropsychiatric disorders (e.g., depression, suicidal ideation, dysthymia) only when in a stable condition with or without medication (44,52). A large meta-analysis of randomized controlled trials did not find relevant adverse effects of varenicline on cardiovascular safety that had previously been considered problematic (53). This finding is especially relevant for transplant recipients, who are already at an increased risk for cardiovascular events due to their immunosuppressive regimen (54). Varenicline is reported to have a low interaction potential (to date reported to be limited to cimetidine) (55). Although this has not specifically been studied in transplant recipients this lack of pharmacological interactions in the context of polymedicated transplant recipients may be considered an advantage for smoking cessation interventions in this population.
The most important side effect of bupropion, a norepinephrine-dopamine reuptake inhibitor (NDRI) and nicotinic antagonist, is an increased risk for epileptic seizures (19). In addition to drug interactions, some common drugs in TPL medicine (e.g., cyclosporine, tacrolimus, prednisone and beta-lactam antibiotics) can also lower seizure threshold (56). Another contraindication for bupropion is a decreased liver function. Patients with CF, the third most common underlying diagnosis for lung TPL after COPD and ILD worldwide, commonly have hepatobiliary dysfunction (i.e., biliary cirrhosis, cholelithiasis, cholestasis and portal hypertension) (5,10,57). Additionally, macrolides and sulfonamides, being well-established antibiotics also used post-transplant, are associated with acute and chronic drug induced liver injury (DILI), characterized by continued elevation (>2× the upper limit of normal) in alanine aminotransferase (ALT) (58,59). In case reports, the use of bupropion has also been linked to a reduced clinically effective concentration of cyclosporine (60).
Electronic cigarettes (e-cigarettes), are increasingly popular devices that produce an aerosol by heating a liquid containing solvents (glycerin, propylene glycol), flavorings and nicotine (61). The efficacy of e-cigarettes as a smoking cessation or reduction intervention remains uncertain due to the limited data available from randomized controlled trials. Dual consumption of tobacco cigarettes and e-cigarettes is a common finding, thus, making e-cigarettes a questionable smoking cessation aid for transplant recipients, who should achieve immediate and complete tobacco abstinence (62,63).
Cytisine acts, like varenicline, as a partial agonist of nicotinic acetylcholine receptors. Despite its proven efficacy and favorable cost-benefit ratio, its application in clinical settings, outside resource poor countries, is still very limited (64). In 2014, Walker et al. conducted a large noninferiority trial comparing cytisine versus NRT for smoking cessation (65). In this study, self-reported continuous abstinence from smoking at 1 month was reported in 40% of participants receiving cytisine (264 of 655) and 31% of participants receiving NRT (203 of 655) (95% CI of difference: 4.2–14.5). A main limitation of this trial remains the nicotine abstinence assessment relying only on self-report, instead of cotinine or exhaled CO measurements. In certain transplant patients, in whom varenicline or bupropion may be contraindicated, cytisine may be considered an alternative. However, studies supporting the effectiveness and safety of cytisine for smoking cessation in transplant recipients are missing.
Conclusion and future directions
Smoking resumption is an underestimated and under investigated issue in TPL medicine. Systematic screening of nicotine consumption based on quantitative cotinine measurements should be implemented in routine follow-up visits. As alternative, exhaled CO may be considered. Self-report or relying on clinical suspicion have a low sensitivity for tobacco detection and are inadequate methods without a systematic cotinine screening.
An increasing number of evidence suggests that smoking within 6 months prior to listing for TPL is a marker of poor outcome and may be associated with posttransplant smoking resumption. These findings challenge the 6-month abstinence rule in TPL enrollment of thoracic transplant recipients. However, instead of only considering prolonging the abstinence interval, more effort and resources should be invested in intensive smoking cessation interventions for patients with severe and end-stage lung diseases as routine standard of care. Other factors associated with smoking resumption are underlying diagnosis (COPD, emphysema) and ESHS. This highlights the importance of involving the patient’s family in the smoking cessation programs prior to TPL. Future research should focus on the pre-transplant psychological assessment to better identify and understand high-risk patient profiles that are strongly associated with smoking resumption after TPL.
Due to the side effect profile and possible interactions, an experienced TPL physician familiar with both pharmacological and behavioral smoking cessation interventions should ideally provide pharmacological smoking cessation interventions for TOT recipients. Future clinical trials should assess safety, efficiency and tolerance of pharmacological smoking cessation drugs in TOT. If not contraindicated, NRT and varenicline should be preferred over bupropion based on efficacy and potential for interactions.
Patients and their relatives require access to professional smoking cessation interventions even long before being assessed for TOT, since a long abstinence period prior to TPL appears to be beneficial for relapse prevention.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bilano V, Gilmour S, Moffiet T, et al. Global trends and projections for tobacco use, 1990–2025: an analysis of smoking indicators from the WHO Comprehensive Information Systems for Tobacco Control. Lancet 2015;385:966-76. [Crossref] [PubMed]
- Mathers CD, Loncar D. Projections of Global Mortality and Burden of Disease from 2002 to 2030. PLoS Med 2006;3. [Crossref] [PubMed]
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014.
- Fagerström K. The epidemiology of smoking: health consequences and benefits of cessation. Drugs 2002;62 Suppl 2:1-9. [Crossref] [PubMed]
- Lund LH, Khush KK, Cherikh WS, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Heart Transplantation Report—2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant 2017;36:1037-46. [Crossref] [PubMed]
- Villalon E. Overcoming Challenges. Prog Transplant 2016;26:260-2. [Crossref] [PubMed]
- Reeb J, Keshavjee S, Cypel M. Expanding the lung donor pool: advancements and emerging pathways. Curr Opin Organ Transplant 2015;20:498-505. [Crossref] [PubMed]
- Mehra MR, Kobashigawa J, Starling R, et al. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates-2006. J Heart Lung Transplant 2006;25:1024-42. [Crossref] [PubMed]
- Kreider M, Kotloff RM. Selection of Candidates for Lung Transplantation. Proc Am Thorac Soc 2009;6:20-7. [Crossref] [PubMed]
- Orens JB, Estenne M, Arcasoy S, et al. International Guidelines for the Selection of Lung Transplant Candidates: 2006 Update—A Consensus Report From the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006;25:745-55. [Crossref] [PubMed]
- Corbett C, Armstrong MJ, Neuberger J. Tobacco smoking and solid organ transplantation. Transplantation 2012;94:979-87. [Crossref] [PubMed]
- Duerinckx N, Burkhalter H, Engberg SJ, et al. Correlates and Outcomes of Posttransplant Smoking in Solid Organ Transplant Recipients: A Systematic Literature Review and Meta-Analysis. Transplantation 2016;100:2252-63. [Crossref] [PubMed]
- Sampaio MS, Cho YW, Qazi Y, et al. Posttransplant malignancies in solid organ adult recipients: an analysis of the U.S. National Transplant Database. Transplantation 2012;94:990-8. [Crossref] [PubMed]
- Choi YH, Leung AN, Miro S, et al. Primary bronchogenic carcinoma after heart or lung transplantation: radiologic and clinical findings. J Thorac Imaging 2000;15:36-40. [Crossref] [PubMed]
- Costanzo MR, Eisen HJ, Brown RN, et al. Are there specific risk factors for fatal allograft vasculopathy? An analysis of over 7,000 cardiac transplant patients. J Heart Lung Transplant 2001;20:152. [Crossref] [PubMed]
- Arcasoy SM, Hersh C, Christie JD, et al. Bronchogenic carcinoma complicating lung transplantation. J Heart Lung Transplant 2001;20:1044-53. [Crossref] [PubMed]
- Wan F, Dai H, Zhang S, et al. Cigarette smoke exposure hinders long-term allograft survival by suppressing indoleamine 2, 3-dioxygenase expression. Am J Transplant 2012;12:610-9. [Crossref] [PubMed]
- Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther 2008;83:531-41. [Crossref] [PubMed]
- Wilkes S. The use of bupropion SR in cigarette smoking cessation. Int J Chron Obstruct Pulmon Dis 2008;3:45-53. [Crossref] [PubMed]
- Babb S, Malarcher A, Schauer G, et al. Quitting Smoking Among Adults — United States, 2000–2015. MMWR Morb Mortal Wkly Rep 2017;65:1457-64. [Crossref] [PubMed]
- Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol 2009;49:57-71. [Crossref] [PubMed]
- Warnier MJ, van Riet EES, Rutten FH, et al. Smoking cessation strategies in patients with COPD. Eur Respir J 2013;41:727-34. [Crossref] [PubMed]
- Benowitz NL, Iii P, Ahijevych K, et al. Biochemical verification of tobacco use and cessation. Nicotine Tob Res 2002;4:149-59. [Crossref] [PubMed]
- Stitzer ML, Bigelow GE. Contingent reinforcement for reduced carbon monoxide levels in cigarette smokers. Addict Behav 1982;7:403-12. [Crossref] [PubMed]
- Jaffe JH, Kanzler M, Friedman L, et al. Carbon monoxide and thiocyanate levels in low tar/nicotine smokers. Addict Behav 1981;6:337-43. [Crossref]
- van der Aalst CM, de Koning HJ. Biochemical verification of the self-reported smoking status of screened male smokers of the Dutch–Belgian randomized controlled lung cancer screening trial. Lung Cancer 2016;94:96-101. [Crossref] [PubMed]
- Ruttens D, Verleden SE, Goeminne PC, et al. Smoking resumption after lung transplantation: standardised screening and importance for long-term outcome. Eur Respir J 2014;43:300-3. [Crossref] [PubMed]
- Evangelista L, Ter-Galstanyan A, Moser DK, et al. Smoking Among Women Following Heart Transplantation: Should We Be Concerned? Prog Cardiovasc Nurs 2009;24:119-23. [Crossref] [PubMed]
- Evon DM, Burker EJ, Sedway JA, et al. Tobacco and alcohol use in lung transplant candidates and recipients. Clin Transplant 2005;19:207-14. [Crossref] [PubMed]
- De Geest S, Dobbels F, Fluri C, et al. Adherence to the therapeutic regimen in heart, lung, and heart-lung transplant recipients. J Cardiovasc Nurs 2005;20:S88-98. [Crossref] [PubMed]
- Bauldoff GS, Holloman CH, Carter S, et al. Cigarette smoking following lung transplantation: effects on allograft function and recipient functional performance. J Cardiopulm Rehabil Prev 2015;35:147-53. [Crossref] [PubMed]
- Zmeškal M, Králíková E, Kurcová I, et al. Continued Smoking In Lung Transplant Patients: A Cross Sectional Survey. Zdr Varst 2015;55:29-35. [Crossref] [PubMed]
- Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2010.
- van der Heide F, Dijkstra G, Porte RJ, et al. Smoking behavior in liver transplant recipients. Liver Transpl 2009;15:648-55. [Crossref] [PubMed]
- Herrero JI, Pardo F, D'Avola D, et al. Risk factors of lung, head and neck, esophageal, and kidney and urinary tract carcinomas after liver transplantation: the effect of smoking withdrawal. Liver Transpl 2011;17:402-8. [Crossref] [PubMed]
- Watt KD, Pedersen RA, Kremers WK, et al. Long-term probability of and mortality from de novo malignancy after liver transplantation. Gastroenterology 2009;137:2010-7. [Crossref] [PubMed]
- Botha P, Peaston R, White K, et al. Smoking after cardiac transplantation. Am J Transplant 2008;8:866-71. [Crossref] [PubMed]
- Nägele H, Kalmár P, Rödiger W, et al. Smoking after heart transplantation: an underestimated hazard? Eur J Cardiothorac Surg 1997;12:70-4. [Crossref] [PubMed]
- Vos R, De Vusser K, Schaevers V, et al. Smoking resumption after lung transplantation: a sobering truth. Eur Respir J 2010;35:1411-3. [Crossref] [PubMed]
- Basile A, Bernazzali S, Diciolla F, et al. Risk factors for smoking abuse after heart transplantation. Transplant Proc 2004;36:641-2. [Crossref] [PubMed]
- Mehra MR, Uber PA, Prasad A, et al. Recrudescent Tobacco Exposure Following Heart Transplantation: Clinical Profiles and Relationship with Athero-Thrombosis Risk Markers. Am J Transplant 2005;5:1137-40. [Crossref] [PubMed]
- Rea JB, Reidy MF. If there is smoke is there fire? Smoking recidivism after lung transplant. J Heart Lung Transplant 2006;25:S119-20. [Crossref]
- Schiele BC, Baker AB, Hathaway SR. The Minnesota multiphasic personality inventory. Lancet 1943;63:292-7.
- Barboza JL, Patel R, Patel P, et al. An update on the pharmacotherapeutic interventions for smoking cessation. Expert Opin Pharmacother 2016;17:1483-96. [Crossref] [PubMed]
- Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev 2013. [PubMed]
- Stead LF, Bergson G, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev 2008. [PubMed]
- Stead LF, Hartmann-Boyce J, Perera R, et al. Telephone counselling for smoking cessation. Cochrane Database Syst Rev 2013. [PubMed]
- Lindson-Hawley N, Thompson TP, Begh R. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev 2015. [PubMed]
- Vrzal R, Zenata O, Bachleda P, et al. The effects of drugs with immunosuppressive or immunomodulatory activities on xenobiotics-metabolizing enzymes expression in primary human hepatocytes. Toxicol In Vitro 2015;29:1088-99. [Crossref] [PubMed]
- Silagy C, Lancaster T, Stead L, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2004. Update in: Nicotine replacement therapy for smoking cessation. [Cochrane Database Syst Rev. 2008]. [PubMed]
- Jorenby DE, Hays TJ, Rigotti NA, et al. Efficacy of Varenicline, an α4β2 Nicotinic Acetylcholine Receptor Partial Agonist, vs Placebo or Sustained-Release Bupropion for Smoking Cessation: A Randomized Controlled Trial. JAMA 2006;296:56-63. [Crossref] [PubMed]
- Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016;387:2507-20. [Crossref] [PubMed]
- Sterling LH, Windle SB, Filion KB, et al. Varenicline and Adverse Cardiovascular Events: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc 2016;5. [Crossref] [PubMed]
- Graham DJ, By K, McKean S, et al. Cardiovascular and mortality risks in older Medicare patients treated with varenicline or bupropion for smoking cessation: an observational cohort study. Pharmacoepidemiol Drug Saf 2014;23:1205-12. [Crossref] [PubMed]
- Faessel HM, Obach SR, Rollema H, et al. A Review of the Clinical Pharmacokinetics and Pharmacodynamics of Varenicline for Smoking Cessation. Clin Pharmacokinet 2010;49:799-816. [Crossref] [PubMed]
- Hitchings AW. Drugs that lower the seizure threshold. Adverse Drug React Toxicol Rev 2016;298:1151-4.
- Parisi GF, Di Dio G, Franzonello C, et al. Liver Disease in Cystic Fibrosis: an Update. Hepat Mon 2013;13. [Crossref]
- Stine JG, Chalasani N. Chronic liver injury induced by drugs: a systematic review. Liver Int 2015;35:2343-53. [Crossref] [PubMed]
- Chalasani N, Bonkovsky HL, Fontana R, et al. Features and Outcomes of 899 Patients with Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology 2015;148:1340-52. [Crossref] [PubMed]
- Lewis BR, Aoun SL, Bernstein GA, et al. Pharmacokinetic Interactions between Cyclosporine and Bupropion or Methylphenidate. J Child Adolesc Psychopharmacol 2001;11:193-8. [Crossref] [PubMed]
- Dinakar C, O'Connor GT. The Health Effects of Electronic Cigarettes. N Engl J Med 2016;375:1372-81. [Crossref] [PubMed]
- Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med 2016;4:116-28. [Crossref] [PubMed]
- McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev 2014. [PubMed]
- Aveyard P, West R. Cytisine and the failure to market and regulate for human health. Thorax 2013;68:989. [Crossref] [PubMed]
- Walker N, Howe C, Glover M, et al. Cytisine versus Nicotine for Smoking Cessation. N Engl J Med 2014;371:2353-62. [Crossref] [PubMed]