Predicting prognosis of post-chemotherapy patients with resected IIIA non-small cell lung cancer
Introduction
Lung cancer remains the leading cause of cancer mortality in the world (1,2). Prevalence of lung cancer is extremely increasing in developing countries especially in China (2). With the help of chest computed tomography (CT) and some other techniques, more curable-stage lung cancers including a subset of stage III cancers are detected and they are well completely resected (3,4). It is well known that the seventh tumor, node, metastasis (TNM) staging system is widely used for staging and survival predicting based on T and M descriptors and in the nodal map (5). Although this system achieved satisfied accuracy in predicting survival outcomes, additional information, if added, can help increase the prediction performance of the model, especially in postoperative patients. So it is believed that there are some other independent prognostic factors such as patient-related and treatment-related factors which contribute to the individualized prediction of relapse and survival (6).
Nomograms have been widely developed in the majority of cancers for more individualized prognostic information of recurrence and survival outcome based on a combination of variables compared with traditional TNM staging system (7-10). They can also be used for predicting long-term survival outcome after surgery precisely (11). For resected non-small-cell lung cancer (NSCLC), a well-established nomogram can successfully provide an individual prediction of overall survival (OS) (11). For advanced NSCLC, nomogram can also help physicians to predict short-term survival before treatment and make optimal clinical decisions (10).
In this study, several clinical factors such as sex, age, smoke status, histology, and some tumor-related factors are selected and nomograms are developed for predicting the recurrence and survival of postoperative chemotherapy patients with resected IIIA NSCLC, in order to estimate the recurrence and survival of post-chemotherapy patients with resected IIIA NSCLC more precisely.
Methods
Patients
We retrospectively analyzed a consecutive database of pathological IIIA NSCLC patients treated with postoperative chemotherapy at Fudan University Shanghai Cancer Center between October 2007 and May 2013. All patients were initially treated with surgery according to our treatment strategy (12). The inclusion criteria were: (I) NSCLC under complete resection; (II) pathological IIIA stage according to the seventh edition; (III) underwent postoperative chemotherapy. Exclusion criteria were: (I) small-cell lung cancer (SCLC); (II) tumor metastasis or uncertain origin; (III) cannot under complete resection; (IV) not belong to pathological IIIA stage; (V) not receive chemotherapy; (VI) perioperative death; (VII) receive neoadjuvant therapy.
For all these qualified patients, we conduct a well-performed follow up focusing on the relevant information including: patient-related data (sex, age, smoke history, comorbidity, family history); treatment-related data (resection extent, post radiotherapy, post targeted therapy); tumor-related data (tumor size, N stage, count of positive lymph nodes in N1 group, count of positive lymph nodes in N2 group (8), histology and EGFR mutation). Two pathologists (Y Li and X Shen) independently confirmed the histology of resected tumor samples and count and group of lymph node samples.
Patients’ relapse-free survival (RFS) was defined as the date of surgery to the date of relapse and patients’ OS is defined from the date of surgery to the date of death or last contact. Patients were regularly followed every 3 months during the first 3 years postoperatively, and every 6 months during 3 to 5 years postoperatively. Enhanced chest CT scan and abdominal ultrasonography were recommended for every clinic visit. Brain magnetic resonance imaging and bone scanning were recommended every 6 months. If tumor recurrence was suspected, pathologic confirmation was usually obtained. Patients whose tumor recurred in organs that were impossible to obtain biopsies were confirmed by the multidisciplinary tumor board, decisions were made mainly based on the dynamic changes in imaging or liquid biopsy.
Statistical methods
Cox proportional hazards regression was performed for univariable analyses of RFS and OS. Only those prognostic variables which achieved the significance at P<0.05 can be selected into the multivariable analyses of Cox proportional hazards regression (10,11). Both univariable and multivariable analyses of cox proportional hazards regression were conducted by SPSS 20.0 for windows.
Nomograms were developed by R 3.2.2 using “rms” package after analyzing the multivariable cox regression (13,14), and concordance index (C-index) was calculated to quantify the discrimination between outcomes and models (14-16). Values of C-index 0.5 and 1 indicate poor and perfect discriminative ability, respectively (16). Next, calibration curves were performed including 3-year and 5-year of RFS and OS, to identify the difference between predicted model and observed data (17). Finally, we tried to conduct risk stratification according to risk scores calculated by nomograms of RFS and OS. Risk stratifications were presented by Kaplan-Meier survival curves and Log-rank test (11).
Results
Clinicopathological characteristics and survival of the patients
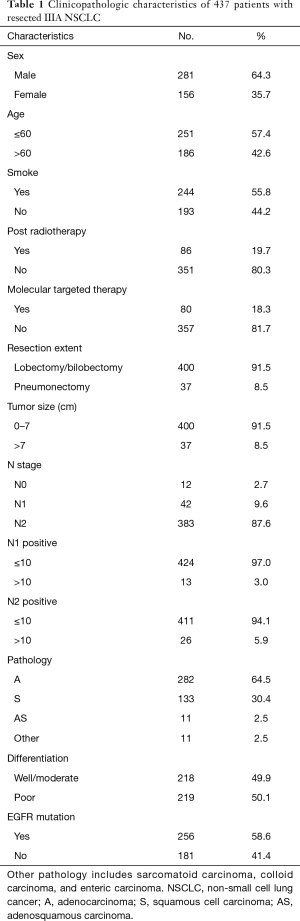
From October 2007 to May 2013, there were 437 qualified patients included in our study. Among them, 281 (64.3%) patients were male, and 156 (35.7%) patients were female. There were 244 (55.8%) smokers and 186 (42.6%) non-smokers. In this study, 86 (19.7%) patients received post radiotherapy (patients with multi-station N2 involvement and high positive lymph node ratio (>50%) were suggested to receive adjuvant radiotherapy). And 80 (18.3%) patients received post targeted therapy. Most of the patients (87%) finished four to six full cycles of platinum-based chemotherapy (pemetrexed plus cisplatin for lung adenocarcinoma, and gemcitabine plus cisplatin for lung squamous cell carcinoma as first-line chemotherapy). The rest of the patients discontinued chemotherapy because of intolerance or serious complications. The clinicopathological characteristics were summarized in Table 1. With a median follow-up of 25.4 months (range 0.3 to 81.9 months), 303 (69.3%) patients had experienced recurrence and 182 (41.6%) patients had died. The median RFS is 13.1 (95% CI: 11.1–15.1) months, and the median OS is 47.7 (95% CI: 37.8–57.6) months.
Full table
Cox proportional hazards regression of univariable and multivariable analyses
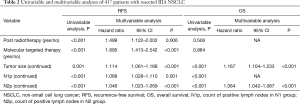
In the first step, we put all the clinicopathological variables including sex, age, smoke status, post radiotherapy, post targeted therapy, resection extent, tumor size, N stage, pathology, tumor differentiation, and EGFR mutation in univariable cox regression analyses of RFS and OS, respectively. We also counted the number of positive lymph nodes in N1 group and N2 group, and performed univariable analyses. In RFS, post radiotherapy (P<0.001), post-targeted therapy (P<0.001), tumor size (P=0.001), N stage (P=0.007), positive lymph node count in N1 group (N1p) (P<0.001) and positive lymph node count in N2 group (N2p) (P<0.001) were significantly related to prognosis. With respect to OS, sex (P=0.010), smoke (P=0.025), tumor size (P<0.001), N1p (P<0.001), N2p (P<0.001), tumor differentiation (P<0.001), pathology (P=0.009), and EGFR mutation (P=0.021) were significantly related to prognosis.
In the second step, all potential prognostic factors selected by univariable analysis were entered into multivariable analysis of cox proportional hazards regression. There were five independent factors (post radiotherapy, post targeted therapy, tumor size, N1p and N2p) left in the RFS model and two (tumor size and N2p) left in the OS model (Table 2).
Full table
Nomogram development
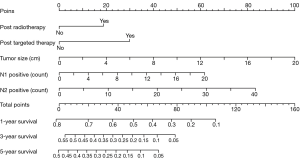
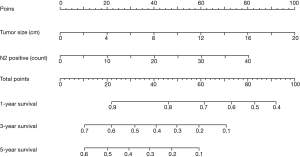
According to the multivariable analyses, all the independent prognostic factors related to RFS and OS were included in building two nomograms, respectively. It was obvious that tumor size made a huge contribution to the prognosis not only in recurrence but also in OS. In nomogram of RFS (Figure 1), N2p showed much more significant influence than N1p. Post radiotherapy and post targeted therapy showed a moderate impact on prognosis. While both tumor size and N2p shared great contribution to prognosis in the nomogram of OS (Figure 2).
Validation and calibration for nomogram
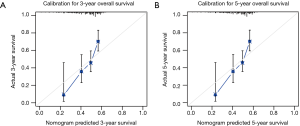
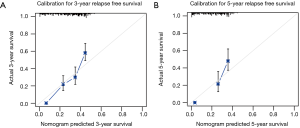
Harrell’s C-index for prediction of RFS was 0.656 (95% CI: 0.626–0.687), and C-index for prediction of OS was 0.651 (95% CI: 0.611–0.691). Calibration curves of 3- and 5-year of RFS and OS were also drawn, which showed moderate agreement between prediction and actual observation, respectively (Figure 3 and Figure S1).
Application of nomogram
Risk score in RFS based on nomogram was calculated by the following equation: RFS score = (19 × post radiotherapy) + (30 × post targeted therapy) + (5 × tumor size) + (3.10 × N1p – 0.05) + (2.08 × N2p – 0.04). And risk score in OS was calculated as follows: OS score = (5 × tumor size) + (2 × N2p).
For example, a 70-year-old male patient, he was pathologically diagnosed as IIIa lung adenocarcinoma after surgery. According to his pathology report, his tumor size was 3.2 cm. There were two positive lymph nodes in N1 group and four in N2 group. He then received post chemotherapy and post targeted therapy. Therefore his RFS score was 60.43 and his OS score was 24. To predict his 5-year relapse free survival probability, we firstly identify the point of 60.43 in the “total points” scale in nomogram of RFS. Then we draw a line perpendicular to this scale down until it meets the “5-year survival” scale. The cross point is approximate 0.09, which means the probability of this patient living free from relapse for 5 years is about 9%.
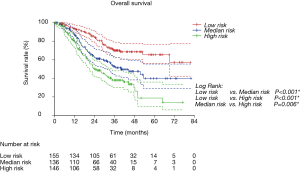
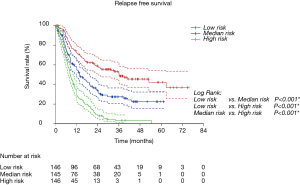
Risk group stratification based on nomogram
We first calculated the risk score for every patient according to nomogram. Then we sorted all patients from small risk scores to large ones. Finally, we divided these sorted patients into three equal parts, named low risk group, median risk group and high risk group. Kaplan-Meier survival curves of RFS and OS were delineated (Figure 4 and Figure S2). It was obvious that different groups have distinct prognoses. Risk score could effectively predict the outcome.
Discussion
Though TNM classification still remains the predominant and powerful indication to the prognosis of NSCLC (18), there are some other prognostic factors such as performance status, sex, age, as well as histology cell type playing an important role in survival prediction (19). And with respect to stage I to stage IIIA NSCLC after surgical treatment, it still holds several prognostic factors in addition to TNM staging (18,20). In recent years, several predicting models have been created for NSCLC (10,21), even for postoperative NSCLC (11). Nomograms have been successfully applied to early-stage and advanced patients, chemo-naive patients, and TKI-treatment patients. Both PFS and OS can be precisely predicted. The emerging nomograms reflect the insufficient of current treatment based on TNM staging system, and the desire of clinicians for individualized treatment of lung cancer. However, predicting model for resected IIIA patients with post-chemotherapy is still scarce. Therefore we tried to develop the nomograms for predicting the recurrence and survival of post-chemotherapy with resected IIIA NSCLC.
After univariable and multivariable analyses, five independent prognostic factors related to relapse free survival and two independent prognostic factors related to OS were selected. And nomograms for RFS and OS were developed. In the nomogram of RFS, tumor size was the predominant factor associated with recurrence, the greater the tumor size was, the higher the risk score was. The count of the positive lymph nodes in N2 group was more important than that in N1 group. Post targeted therapy and post radiotherapy showed a moderate influence in RFS. Several RCTs such as V-15-3 trial (22), BR 19 trial (23) and RADIANT trial (24), showed that there was no survival benefit from postoperative targeted therapy and there was even detriment for those mutation-unselected patients. In our study, however, we found that post targeted therapy might contribute to early recurrence. Post radiotherapy also seemed detrimental to RFS in our study, which was conflicted with recent studies for advocating postoperative radiotherapy in resected pN2 NSCLC (25,26). The contradiction mainly resulted from different endpoints. We used relapse free survival in our study while those studies focused on OS. Furthermore, N stage for post-radiotherapy in our study was not stratified since most of the patients were N2 disease, which might also contribute to a different conclusion. In the nomogram of OS, neither post radiotherapy nor post targeted therapy contributed to prognosis. Only tumor size and count of the positive lymph nodes in N2 group could independently predict outcomes. Our data demonstrated that only tumor-related factors affected patients’ OS. Adjuvant treatments could affect the recurrence, the impact was limited. Radiotherapy or targeted therapy has been proven efficient in advanced NSCLC. However, in resected stage IIIA patients, in view of the intrinsic heterogeneity of this population, the effectiveness of these treatments is yet to be verified in clinical trials.
Validation of nomograms were performed, and C-index was 0.656 (95% CI: 0.626–0.687) for RFS and 0.651 (95% CI: 0.611–0.691) for OS, respectively. These results indicated a moderate concordance between the predicting and actual RFS and OS. We could also found the moderate concordance in calibration curves of RFS and OS (Figure 3 and Figure S1). Furthermore, we made risk stratification according to risk scores calculated by nomograms. Subgroups were delineated through Kaplan-Meier survival curves, with significant p values in different groups for RFS and OS (Figure 4 and Figure S2). So, the risk groups stratified by different risk scores were able to indicate different recurrence and survival outcomes. This method could make doctors easier to estimate the prognosis of post-chemotherapy patients with resected IIIA NSCLC and decide different and optimal clinical decisions.
It was also notable that what the clinical stage was of these patients in our study before surgery. In our hospital, every patient received preoperative tests including chest CT scan/chest X-ray, brain MRI, abdominal CT or ultrasonography, radionuclide bone scan/PET-CT and bronchoscopy to assess clinical stage. For patients suspicious of mediastinal lymph node metastasis, EBUS-TBNA or other invasive examination was performed. Patients whose tumors were considered initially resectable were selected for surgery according to our treatment strategy (12). These patients were either in cN0–1/pN2 or minimal N2 status by imaging evaluation preoperatively. We routinely performed lobectomy and mediastinal lymph node dissection as a standard operation. Only in patients with negative CT scan findings and positive pathological findings in mediastinal lymph node, their clinical stages and pathological stages were inconsistent.
Still, there were several limitations and deficiencies in our study. First, it was a retrospective analysis of a limited number of patients. Second, some of the potential parameters such as vascular invasion, subgroups of N2, were not analyzed in our study (27). Third, lack of external validation was the major limitation to our nomograms. Fourth, Patients included in our analyses started from 2007. Great changes occurred in treatment pattern in recent years. Treatment strategy which was optimal ten years ago might not remain optimal at present. For example, the current immunotherapy which had significant therapeutic effects was not included in our study. And this inevitably influenced the conclusion of our study. Further great efforts on precise follow-ups, careful statistical analyses were needed to make better improvement of our models.
Conclusions
In this study, we developed nomograms of RFS and OS for predicting the recurrence and survival of post-chemotherapy patients with resected IIIA NSCLC. C-indexes were calculated to validate those models. With the help of these nomograms, clinicians could more easily estimate the prognosis of post-chemotherapy patients with resected IIIA NSCLC and choose optimal clinical decisions for individuals.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation of China (81330056, 81472173, 81401891, 81572253, and 81372525), the Key Project of Science and Technology Commission of Shanghai Municipality (JGGG1302).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of the Fudan University Shanghai Cancer Center (No. 090977-1) and written informed consent was obtained from all patients.
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [Crossref] [PubMed]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Christensen JD, Chiles C. Low-Dose Computed Tomographic Screening for Lung Cancer. Clin Chest Med 2015;36:147-60. [Crossref] [PubMed]
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Non- Small Cell Lung Cancer. V 5. 2017.
- Saji H, Tsuboi M, Shimada Y, et al. A Proposal for Combination of Total Number and Anatomical Location of Involved Lymph Nodes for Nodal Classification in Non-small Cell Lung Cancer. Chest 2013;143:1618-25. [Crossref] [PubMed]
- Gospodarowicz MK, O’Sullivan B, Sobin LH. Prognostic factors in cancer. 3rd edn. New Jersey, USA: Wiley-Liss, 2006.
- Wang Y, Li J, Xia Y, et al. Prognostic Nomogram for Intrahepatic Cholangiocarcinoma After Partial Hepatectomy. J Clin Oncol 2013;31:1188-95. [Crossref] [PubMed]
- International Bladder Cancer Nomogram Consortium, Bochner BH, Kattan MW, et al. Postoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancer. J Clin Oncol 2006;24:3967-72. [Crossref] [PubMed]
- Motzer RJ, Bukowski RM, Figlin RA, et al. Prognostic Nomogram for Sunitinib in Patients With Metastatic Renal Cell Carcinoma. Cancer 2008;113:1552-8. [Crossref] [PubMed]
- Hoang T, Xu R, Schiller JH, et al. Clinical Model to Predict Survival in Chemonaive Patients With Advanced Non-Small-Cell Lung Cancer Treated With Third-Generation Chemotherapy Regimens Based on Eastern Cooperative Oncology Group Data. J Clin Oncol 2005;23:175-83. [Crossref] [PubMed]
- Liang W, Zhang L, Jiang G, et al. Development and Validation of a Nomogram for Predicting Survival in Patients With Resected Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:861-9. [Crossref] [PubMed]
- Zheng D, Ye T, Hu H, et al. Upfront surgery as first-line therapy in selected patients with stage IIIA non-small cell lung cancer. J Thorac Cardiovasc Surg 2018;155:1814-1822.e4. [Crossref] [PubMed]
- Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008;26:1364-70. [Crossref] [PubMed]
- Harrell FE Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis (Springer Series in Statistics). New York, NY: Springer, 2001.
- van Gijn W, van Stiphout RG, van de Velde CJ, et al. Nomograms to predict survival and the risk for developing local or distant recurrence in patients with rectal cancer treated with optional short-term radiotherapy. Ann Oncol 2015;26:928-35. [Crossref] [PubMed]
- Maurichi A, Miceli R, Camerini T, et al. Prediction of Survival in Patients With Thin Melanoma:Results From a Multi-Institution Study. J Clin Oncol 2014;32:2479-85. [Crossref] [PubMed]
- Ogura K, Fujiwara T, Yasunaga H, et al. Development and External Validation of Nomograms Predicting Distant Metastases and Overall Survival After Neoadjuvant Chemotherapy and Surgery for Patients With Non-metastatic Osteosarcoma: A Multi-Institutional Study. Cancer 2015;121:3844-52. [Crossref] [PubMed]
- Chansky K, Sculier JP, Crowley JJ, et al. The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathological TNM stage in surgically managed non-small cell lung cancer. J Thorac Oncol 2009;4:792-801. [Crossref] [PubMed]
- Sculier JP, Chansky K, Crowley JJ, et al. The impact of additional prognostic factors on survival and their relationship with the anatomical extent of disease as expressed by the 6th edition of the TNM classification of malignant tumors and the proposals for the 7th edition. J Thorac Oncol 2008;3:457-66.
- Mitra R, Lee J, Jo J, et al. Prediction of Postoperative Recurrence-Free Survival in Non-Small Cell Lung Cancer by Using an Internationally Validated Gene Expression Model. Clin Cancer Res 2011;17:2934-46. [Crossref] [PubMed]
- Keam B, Kim DW, Park JH, et al. Nomogram Predicting Clinical Outcomes in Non-small Cell Lung Cancer Patients Treated with Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors. Cancer Res Treat 2014;46:323-30. [Crossref] [PubMed]
- Tsuboi M, Kato H, Nagai K, et al. Gefitinib in the adjuvant setting: safety results from a phase III study in patients with completely resected non-small cell lung cancer. Anticancer Drugs 2005;16:1123-8. [Crossref] [PubMed]
- Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected nonsmall-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol 2013;31:3320-6. [Crossref] [PubMed]
- Kelly K, Altorki NK, Eberhardt WEE, et al. A randomized, double-blind phase 3 trial of adjuvant erlotinib (E) versus placebo (P) following complete tumor resection with or without adjuvant chemotherapy in patients (pts) with stage IB-IIIA EGFR positive (IHC/FISH) non-small cell lung cancer (NSCLC): RADIANT results. J Clin Oncol 2014;32:abstr 7501.
- Mikell JL, Gillespie TW, Hall WA, et al. Postoperative Radiotherapy is Associated with Better Survival in Non-Small Cell Lung Cancer with Involved N2 Lymph Nodes Results of an Analysis of the National Cancer Data Base. J Thorac Oncol 2015;10:462-71. [Crossref] [PubMed]
- Burdett S, Rydzewska L, Tierney JF, et al. PORT Meta-analysis Trialist Group. A closer look at the effects of postoperative radiotherapy by stage and nodal status: updated results of an individual participant data meta-analysis in non-small-cell lung cancer. Lung Cancer 2013;80:350-2. [Crossref] [PubMed]
- Ichinose J, Murakawa T, Hino H, et al. Prognostic Impact of the Current Japanese Nodal Classification on Outcomes in Resected Non -small Cell Lung Cancer. Chest 2014;146:644-9. [Crossref] [PubMed]