Preoperative peak expiratory flow (PEF) for predicting postoperative pulmonary complications after lung cancer lobectomy: a prospective study with 725 cases
Introduction
Lung cancer, as a leading cause of cancer-related deaths worldwide, has been one of the most health-threatening and death-causing diseases to humans with the morbidity and mortality rate ranking first in China (1-5). Among various therapeutic methods aiming at curing the disease, surgery is still the primary or optimal strategy, especially for intermediate-stage patients with pre-malignant or early lesions which are amenably resectable (6). Postoperative pulmonary complications (PPCs) are deemed to be strongly correlated to short- and long-term survival after lung cancer surgery (5,7,8). Hence, the preoperative state of candidates waiting for lung cancer surgery and predictive risks for PPCs with up-to-date data is urgently needed to be investigated, helping to set realistic expectations for perioperative interventions, therapies and care.
Resent years, some predictive factors of PPCs after lung resection, for example, forced expiratory volume in 1 s (FEV1) or 6-min walk distance (6-MWD), have been deeply investigated, aiming at better assessing the risk of PPCs preoperatively (8-15). peptide level peak expiratory flow (PEF), which is defined as the maximum flow achieved during expiration delivered with maximal force starting from maximal lung inflation, has been investigated as a risk assessment tool aiming at old populations. Recent years several researches have been performed to investigate associations of PEF with long-term cause-specific mortality, for it has cross-sectionally associations with health status as well as physical and cognitive function (16-20). However, few researches were conducted to validate the effectiveness of PEF to predict the occurrence of PPCs after lung cancer lobectomy.
Based on this, we set this prospective study to examine the correlation between PEF and clinical variables in lung cancer patients undergoing lung lobectomy.
Methods
Ethical review
This protocol has been approved by the university’s clinical trials and biomedical ethics committee (No. 2016-121). The WHO registering number is ChiCTR-COC-17010720. We declared that the research was adhered the tenets of the Declaration of Helsinki, with written informed consent obtained from the patients.
Patients
Records of consecutive patients who diagnosed with non-small cell lung cancer (NSCLC) undergoing lung cancer lobectomy at West China Hospital of Sichuan University, between March 1st, 2017 and December 31th, 2017 were involved. Inclusive criteria were listed as follow: (I) diagnosed as NSCLC; (II) undergoing lung cancer lobectomy; (III) age between 40–85 years; (IV) with agreement of informed consent. Finally, data of 725 patients were finally included and analyzed. All patients were received similar routine perioperative preparation or care, including early mobilization, ambulation and breathing exercise by the specialized nurses. The pathological stage was determined based on the eighth edition of the TNM staging system for lung cancer (21,22). Preoperative pulmonary functions, including forced vital capacity (FVC), FEV1 and PEF, were routinely measured at the lung function laboratory of the hospital before the operation.
PPCs
Based on the STS/ESTS complication definition (23), categories and criteria of the PPCs experienced by the patients were as follows: (I) atelectasis documented clinically or radiographically; (II) pneumonia defined according to the criteria including: new or progressive and persistent infiltrate, consolidation or cavitation found by chest radiographs and at least one of the following must be met: fever (>38 °C) without other recognized reasons; leukopenia (<4,000 WBC/mm3) or leukocytosis (<12,000 WBC/mm3); for patients >70 years old, change in mental status with: purulent sputum or change in character, respiratory secretions increasing or needing suction; onset or worsening symptoms (dyspnea, tachypnea, e.g.,) or clinical signs (rales, bronchial breath sounds, e.g.,); (III) adult respiratory distress syndrome (ARDS); (IV) mechanical ventilation >48 h; (V) air leak >7 days; (VI) reintubation; (VII) back to ICU or needing tracheotomy; (VIII) empyema; (IX) chylothorax/bronchopleural fistula.
Statistics analysis
Continuous variables were presented as the means ± standard deviations (SD) and binary variables as proportions (n, %). Fisher’s exact test, Chi square test, and Student’s t-test were used for comparing variables as appropriate. Univariate and multivariate logistic regression analyses were performed, aiming to investigate potential predictive factors of PPCs and evaluate the predictive significance of PEF value for PPCs. Variables with a P<0.20 in the univariate analysis were involved into the multivariate analysis along with the PEF. A receiver operating characteristic (ROC) curve was performed to evaluate the sensitivity and specificity of PEF value for predicting the occurrence of PPCs in lung cancer patients after lung cancer lobectomy. All results were determined significant at a value of P<0.05. Statistical analyses were conducted via SPSS software v.22.0.
Results
Baseline of the patients’ characteristics
Among the 725 included patients, 144 of them were presented PPCs in 30 days after lobectomy, which were divided into PPCs group. The rates of pneumonia (12.0%, 87/725), atelectasis (5.7%, 41/725) and air leak (5.1%, 37/725) ranked at the top of the categories of PPCs. Details were listed in Table 1.

Full table
The characteristics of PPCs group and non-PPCs group were summarized in Table 1. FEV1 (1.83±0.57 vs. 2.00±0.69 L; P=0.007) and PEF value (294.2±85.1 vs. 344.7±89.6 L/min; P<0.001) were found lower in PPCs group, compared with non-PPCs group. Regarding comorbidities, proportions of COPD (22.2%, 32/144 vs. 14.1%, 82/581; P=0.018) was higher in PPCs group than in non-PPCs group. Additionally, patients in PPCs group had longer length of stay (LOS) including postoperative (7.82±4.83 vs. 4.16±2.50 days; P<0.001), total LOS (13.77±5.29 vs. 9.71±4.41 days; P<0.001) and more total in-hospital expense (51,143.1±12,293.2 vs. 48,603.6±12,636.0 ¥; P=0.030) as well as drug cost (9,959.6±3,966.1 vs. 8,086.7±4,484.8 ¥; P<0.001).
Logistics regression for PPCs
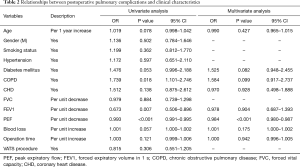
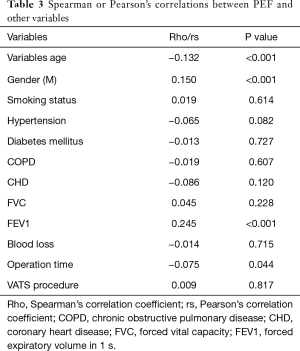
Variables with a P<0.20 in the univariate analysis were into the multivariate analysis, including age (OR: 1.019, P=0.078); diabetes mellitus (OR: 1.476, P=0.053), COPD (OR: 1.739, P=0.018), coronary heart disease (CHD) (OR: 1.512, P=0.138), FEV1 (OR: 0.673, P=0.007), PEF (OR: 0.993; P<0.001), blood loss (OR: 1.001, P=0.057) and operation time (OR: 1.003, P=0.121). By using Multivariate logistic regression analysis, PEF (OR: 0.984, 95% CI: 0.980–0.987, P<0.001) was a significant independent predictors for the occurrence of PPCs. Details were listed in Table 2. Next, factors affecting PEF were examined. Spearman or Pearson’s correlations between PEF and clinical variables of all patients were showed in Table 3. Gender (P<0.001), age (P<0.001), FEV1 value (P<0.001) and operation time (P=0.044) were significantly correlated with PEF.
Full table
Full table
Optimal cutoff of the PEF for predicting PPCs
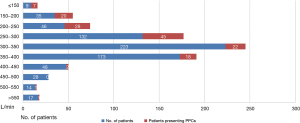
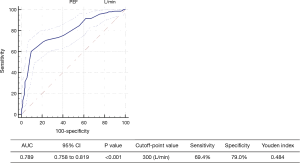
The distribution of PEF in patients with and without PPCs was shown in Figure 1. We selected the optimal cutoff value of the PEF for predicting PPCs based on a ROC curve (Figure 2), with the consideration of balancing the sensitivity and specificity. Hence, we chose a cutoff value of 300 (L/min) (Youden index: 0.484, sensitivity: 69.4%, specificity: 79.0%). Moreover, A PEF ≤300 L/min indicated an 8-fold increase in odds of having PPCs after lung surgery (OR, 8.551, 95% CI: 5.692–12.845, P<0.001).
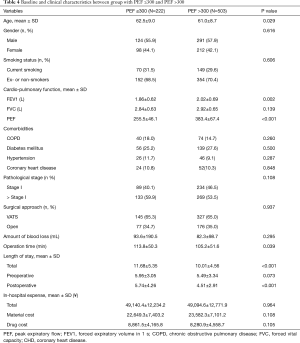
The patients were divided into two groups based on whether the PEF value ≤300 L/min (Table 4). Older age (62.5±9.0 vs. 61.0±8.7 years, P=0.029), lower average FEV1 (1.86±0.62 vs. 2.02±0.69 L, P=0.002), longer operation time (113.8±50.3 vs. 105.2±51.6 min, P=0.039) and postoperative LOS (5.74±4.26 vs. 4.51±2.91 days, P<0.001) as well as total LOS (11.68±5.35 vs. 10.01±4.56 days, P<0.001) were found in the group with PEF value ≤300 L/min. With regard to PPCs rate, patients with PEF value ≤300 L/min had high PPCs rate than those with PEF >300 L/min (45.0%, 100/222 vs. 8.7%, 44/503, P<0.001); meanwhile, pneumonia (24.8%, 55/222 vs. 6.4%, 32/503, P<0.001), atelectasis (9.5%, 21/222 vs. 4.0%, 20/503, P=0.003) and mechanical ventilation >48 h (5.4%, 12/222 vs. 2.4%, 12/503, P=0.036) were higher in the group with PEF value ≤300 L/min (Table 5).
Full table

Full table
Discussion
The significant finding of this prospective study was that for lung cancer patients undergoing lobectomy, preoperative PEF value was significantly lower in patients with PPCs after lobectomy than those who without, and PEF ≤300 L/min was a good predictive parameter in discriminating PPCs and PEF.
Numerous studies related to cause-specific mortality have validated that impaired or poor lung function predicts mortality from other specific conditions including lung cancer, rather than from non-neoplastic respiratory disease. Some variables of lung function, for example, FEV1, traditionally has been considered as the critical component of the functional workup of lung cancer candidates waiting for surgery (13-15), as a reduced FEV1 value is considered to be associated with increased respiratory morbidity and mortality rates for surgical lung cancer patients. In the ACCP guidelines [2013] of Physiologic Evaluation of the Patient with Lung Cancer Being Considered for Resectional Surgery, it is also recommended to evaluate preliminary cardiac-pulmonary function (24). Recent years, several studies has been performed to state PEF as a useful measure of physical functioning and health status in elderly population, which is considered to be an independent predictor of increased health-care utilization. Meanwhile, several other studies concerning the relationships between PEF and subsequent cause–specific mortality also reveal associations with cardiovascular events as well as lung-cancer mortality (16-20,25). PEF, as it can be rapidly and easily measured by an in-expensive and hand-held device, may be potentially useful as an indicator of health status for populations with limited access to healthcare, though it has not be validated (18-20). Meanwhile, the PEF reflects airway patency and resistance, respiratory muscle strength and other aspects of lung function, and reduced PEF was evident in a variety of chronic illnesses, and its validity as a health status measure was confirmed by some studies. Hence, we hypothesized that a low PEF suggested poor respiratory muscle strength and lung function, and may be correlated with the occurrence of PPCs after lung cancer lobectomy.
In our study, among the 725 included patients, 19.8% of them were presented PPCs in 30 days after lobectomy with pneumonia, atelectasis and air leak ranking at the top of the categories of PPCs. The occurrence of PPCs inevitably prolonged hospitalization, slowed the postoperative recovery and rehabilitation, increased the risk of morbidity, as well as both medical and labor cost. Based on the results, patients in PPCs group had longer LOS including postoperative (P<0.001), total LOS (P<0.001) and more total in-hospital expense (P=0.030) as well as drug cost (P≤0.001), revealing the adverse effect the PPCs brought to the patients. Meanwhile, FEV1 (P=0.007) and PEF value (P<0.001) were found lower in PPCs group and via multivariate logistic regression analysis and PEF was significant independent predictors for the occurrence of PPCs. Moreover, when we selected 300 L/min as cutoff point based on Onodera’s original cutoff, with the consideration of balancing the sensitivity and specificity, A PEF ≤300 L/min indicated an 8-fold increase in odds (95% CI: 5.692–12.845, P<0.001) of having PPCs after lung cancer surgery, indicating the potential of a low PEF to predict the occurrence of PPCs after lung cancer lobectomy. Furthermore, when the patients were divided into two groups based on whether the PEF value ≤300 L/min, patients with PEF value ≤300 L/min had high PPCs rate as well as pneumonia, atelectasis and mechanical ventilation >48 h than those with PEF >300 L/min. This would significantly explain the strong correlation between a low PEF and PPCs for surgical lung cancer patients.
There are some limitations which can hardly be avoided including its non-randomized nature and single-center design and potential selection bias. All study participants were selected from March 1st, 2017 and December 31th, 2017 by a small group of surgeons in a single regional center. Secondly, we selected patients who underwent lung cancer lobectomy, which meant that those with other surgical type, such as wedge resection, segmental resection or pneumonectomy were not involved, for excluding the confounder caused by surgical types. But on the other side, selection bias was generated, which inevitably limited the generalization of the conclusions. Furthermore, just like other index of lung function (FEV1, e.g.,), age, sex and height gender may be associated with PEF, which makes it necessary for us to perform further study to investigate. Moreover, in the study, we tried to investigate the correlations between some variables and PEF. However, the relative low values of correlation coefficient suggested the relevant correlations were uncertain. Further study is needed to be performed to deeply discuss the issue. Last not the least, for those patients with low PFE value, more researches should be performed for reducing the relatively high risk of PPCs after surgery. We recommend that more attention should be given to some interventions, for example, perioperative pulmonary rehabilitation which may enhance or improve the lung function, and sequentially lead to reduce PPCs rates.
In conclusion, the study we presented identified a significant correlation between a low PEF value and PPCs in surgical lung cancer patients undergoing lobectomy, indicating a low PEF as independent risk factor for the occurrence of PPCs and a PPC-guided (PEF value ≤300 L/min) risk assessment could be meaningful for the perioperative management of lung cancer candidates waiting for surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the university’s clinical trials and biomedical ethics committee (No. 2016-121) and written informed consent was obtained from all patients.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Boffa DJ, Allen MS, Grab JD, et al. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg 2008;135:247-54. [Crossref] [PubMed]
- Chen W, Zheng RS, Baade PD, et al. Cancer Statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Seely AJ, Ivanovic J, Threader J, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg 2010;90:936-42. [Crossref] [PubMed]
- Andalib A, Ramana-Kumar AV, Bartlett G, et al. Influence of postoperative infectious complications on long-term survival of lung cancer patients: a population-based cohort study. J Thorac Oncol 2013;8:554-61. [Crossref] [PubMed]
- Jazieh AR, Kyasa MJ, Sethuraman G, et al. Disparities in surgical resection of early-stage non-small cell lung cancer. J Thorac Cardiovasc Surg 2002;123:1173-6. [Crossref] [PubMed]
- Brunelli A, Drosos P, Dinesh P, et al. The severity of complications is associated with postoperative costs after lung resection. Ann Thorac Surg 2017;103:1641-6. [Crossref] [PubMed]
- Shiono S, Abiko M, Sato T. Postoperative complications in elderly patients after lung cancer surgery. Interact Cardiovasc Thorac Surg 2013;16:819-23. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-e190S.
- Sawabata N, Nagayasu T, Kadota Y, et al. Risk assessment of lung resection for lung cancer according to pulmonary function: republication of systematic review and proposals by guideline committee of the Japanese association for chest surgery 2014. Gen Thorac Cardiovasc Surg 2015;63:14-21. [Crossref] [PubMed]
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17-41. [Crossref] [PubMed]
- Berry MF, Hanna J, Tong BC, et al. Risk factors for morbidity after lobectomy for lung cancer in elderly patients. Ann Thorac Surg 2009;88:1093-9. [Crossref] [PubMed]
- Schünemann HJ, Dorn J, Grant BJ, et al. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest 2000;118:656-64. [Crossref] [PubMed]
- Wasswa-Kintu S, Gan WQ, Man SF, et al. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: a systematic review and meta-analysis. Thorax 2005;60:570-5. [Crossref] [PubMed]
- Agostini PJ, Lugg ST, Adams K, et al. Risk factors and short-term outcomes of postoperative pulmonary complications after VATS lobectomy. J Cardiothorac Surg 2018;13:28. [Crossref] [PubMed]
- Fragoso CA, Gahbauer EA, Van Ness PH, et al. Peak expiratory flow as a predictor of subsequent disability and death in community-living older persons. J Am Geriatr Soc 2008;56:1014-20. [Crossref] [PubMed]
- Young RP, Hopkins R, Eaton TE. Forced expiratory volume in one second: not just a lung function test but a marker of premature death from all causes. Eur Respir J 2007;30:616-22. [Crossref] [PubMed]
- Vaz Fragoso CA, Gahbauer EA, Van Ness PH, et al. reporting peak expiratory flow in older persons. J Gerontol A Biol Sci Med Sci 2007;62:1147-51. [Crossref] [PubMed]
- Roberts MH, Mapel DW. Limited lung function: impact of reduced peak expiratory flow on health status, health-care utilization, and expected survival in older adults. Am J Epidemiol 2012;176:127-34. [Crossref] [PubMed]
- Smith M, Zhou M, Wang L, et al. Peak flow as a predictor of cause-specific mortality in China: results from a 15-year prospective study of 170000 men. Int J Epidemiol 2013;42:803-15. [Crossref] [PubMed]
- Chansky K, Detterbeck FC, Nicholson AG, et al. The IASLC Lung Cancer Staging Project: External Validation of the Revision of the TNM Stage Groupings in the Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2017;12:1109-21.
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Seder CW, Salati M, Kozower BD, et al. Variation in Pulmonary Resection Practices Between The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons General Thoracic Surgery Databases. Ann Thorac Surg 2016;101:2077-84. [Crossref] [PubMed]
- Detterbeck FC, Mazzone PJ, Naidich DP, et al. Screening for lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e78S-e92S.
- Hegewald MJ, Lefor MJ, Jensen RL, et al. Peak expiratory flow is not a quality indicator for spirometry: peak expiratory flow variability and FEV 1 are poorly correlated in an elderly population. Chest 2007;131:1494-9. [Crossref] [PubMed]


