Risk factors and mortality of adults with lung cancer admitted to the intensive care unit
Introduction
Despite improvements in early diagnosis and appropriate treatments such as chemotherapy, targeted therapy, and even immunotherapy for lung cancer, improvements in long-term survival of patients with lung cancer have been limited, and lung cancer remains a devastating disease (1). It is now the leading cause of cancer-related death worldwide (2,3): it leads to 1.6 million deaths annually (4,5). More than 158,000 patients died from lung cancer in the United States in 2016, which accounted for 27% of all cancer deaths (4,5). Much work needs to be done to improve the outcome of lung cancer patients. In addition to early detection and advanced treatment, intensive care unit (ICU) strategies and treatments are important because ICU admission is not uncommon for lung cancer patients with malignancy-related complications or with underlying comorbidities (6).
Factors associated with poor ICU outcomes for these patients include mechanical ventilation (MV) (7-12), poor pre-event performance status (PS) (10,13,14), high admission Acute Physiologic and Chronic Health Evaluation (APACHE) III scores with vasopressor use (11), and refractory disease (8,10,11,15). However, most of the previous studies were single-center, and their data cannot be generalized across ICUs. Moreover, few studies have examined the risk factors for lung cancer patients admitted to the ICU. Therefore, we investigated the risk factors for admission to the ICU, the infectious complications, organ dysfunction during ICU hospitalizations, and the prognosis after ICU admission among patients with lung cancer.
Methods
Data source
This retrospective, population-based cohort study used Taiwan’s National Health Insurance Research Database (NHIRD). Taiwan’s NHI program is a single-payer compulsory system currently enrolls more than 23 million of the country’s legal residents (>99.7% of Taiwan’s population (16). The NHIRD provides detailed healthcare services information about the clinical visits of each beneficiary. It uses International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic and procedure codes. We used the NHIRD’s lung cancer database, which was constructed from the records of all patients with lung cancer and a catastrophic illness card to select cases for our study. In the NHIRD, biopsy and histological verifications are required before a definite diagnosis of carcinoma can be made and a catastrophic illness card can be obtained. Thus, because we used these criteria, the lung cancer cases selected for our study were considered valid and definite. The lung cancer database is a longitudinal subset of the NHIRD; it contains data related to inpatient and outpatient medical care, diagnoses, surgical procedures, and prescribed medications from 2003 to 2012. The study was approved by the Institutional Review Board (IRB no: 10411-E01) of Chi Mei Medical Center. Because the data used in this study are de-identified, and have been released to the public for research, the IRB waived informed consent.
Case selection and definition
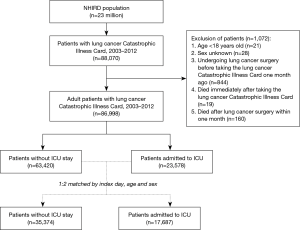
The lung cancer database included 88,070 patients with a lung cancer catastrophic illness card. After excluding patients <18 years old, of unknown sex, who underwent lung cancer surgery just one month before receiving the lung cancer catastrophic illness card, who died immediately after undergoing lung cancer catastrophic illness care, or who died within one month after lung cancer surgery, 86,998 cases were selected for our study: 23,578 had been admitted to the ICU between January 1, 2003, and December 31, 2012. ICU admission was defined according to medical expenditure applications, and the date of ICU admission was defined as the index date. To investigate the risk factor of ICU admission, each case admitted to the ICU was index-date-, age-, and sex-matched with two cases not admitted to the ICU. Finally, the records of 17,687 cases admitted to the ICU and 35,374 cases not admitted to the ICU were selected for analysis (Figure 1).
Baseline variables
To be as comprehensive as possible when adjusting for factors that might affect outcome, we considered the following as potential confounders: sex, age, hospital accreditation, and underlying comorbidities. Comorbidities were defined according to the ICD-9-CM including diabetes mellitus (DM) [ICD-9-CM codes: 250], hyperlipidemia [272], hypertension [401–405], stroke [430–438], chronic obstructive pulmonary disease (COPD) [490–496], chronic liver disease [571], and chronic kidney disease (CKD) [585] within 1 year before their index admission. We also collected information about several other possible confounders—anticancer treatments ≤1 year of ICU admission: surgery, chemotherapy, radiotherapy, and targeted therapy; infectious diseases; and organ dysfunction by using ICD coding.
Endpoint
The primary endpoint of the study was one-year mortality. The definition of mortality was based on the death record from the inpatient claim dataset. Patients were followed from January 1, 2003, until the earliest onset of one of two occurrences, whichever came first: mortality or the end of the study, December 31, 2013.
Statistical analysis
All categorical variables are presented as frequencies with percentages, and the Pearson’s χ2 test or Fisher’s exact test was used to compare the differences between lung cancer patients admitted (ICU+) and not admitted (ICU–) to the ICU. Continuous variables are presented as means ± standard deviation (SD) or median with interquartile range (IQR), and a Student’s t-test or Wilcoxon rank-sum test was used to compare age and time to mortality or follow-up time. In addition, Kaplan-Meier survival curves were used for the 1-year survival rate during the study, and a log-rank test was used to compare risks between ICU+ and ICU– patients. The ratio of 1-year mortality between ICU+ and ICU– patients was estimated using Cox proportional regression models adjusted for age, sex, comorbidities, lung cancer surgery, recent anticancer treatment, infectious diseases, organ dysfunction, and hospital level. A multiple Cox regression analysis was used to find the potential predictors of 1-year mortality among ICU+ patients. SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was used for all analyses. Significance was set at P<0.05. Kaplan-Meier survival curves were plotted using STATA 12 (Stata Corp., College Station, TX, USA).
Results
Clinical characteristics
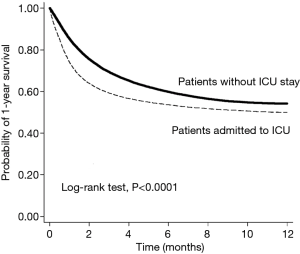
ICU+ patients had more comorbidities—DM, HTN, strokes, COPD, chronic liver disease, and CKD—than did ICU– patients (Table 1). In contrast, ICU– patients had more hyperlipidemia than did ICU+ patients. ICU+ patients were more likely to be treated with chemotherapy, radiotherapy, and targeted therapy than were ICU– patients. In contrast, ICU– patients were more likely to have undergone prior lung cancer surgery than were ICU+ patients. Regarding critical care procedures, ICU+ patients are more likely to receive MV, continuous renal replacement therapy (CRRT), extracorporeal membrane oxygenation (ECMO), and cardiopulmonary resuscitation (CPR) than ICU- patients. ICU+ patients were more frequently treated in a medical center or regional hospital than were ICU– patients. The overall 1-year mortality rate was significantly higher for ICU+ patients than for ICU– patients (49.91% vs. 44.86%, respectively; P<0.0001), and the time to one-year mortality was significantly shorter for ICU+ patients than for ICU– patients (P<0.0001). Kaplan-Meier survival analysis showed that ICU+ patients had a lower 1-year survival rate than did ICU– patients (Log rank test, P<0.001) (Figure 2). In the subgroup analysis, for ICU+ patients remained significant in each subgroup, irrespective of age, sex, comorbidities, recent anticancer treatment, and hospital level (Table 2).
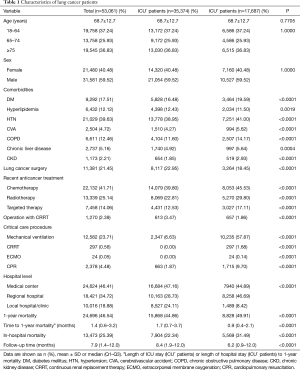
Full table
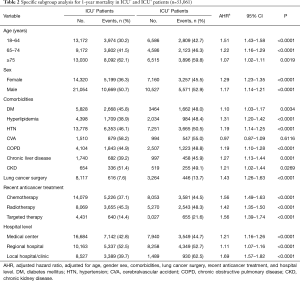
Full table
Complications of ICU+ lung cancer patients
Of the 17,687 ICU+ lung cancer patients, the median length of ICU stay was 4 days (IQR: 2–11 days). More than half of these patients had an infectious disease (n=9,933, 56.16%): respiratory tract infections were most common (n=7,182, 40.61%), and septicemia (n=4,269, 24.14%). Almost as many (n=9,256, 52.33%) had an organ dysfunction: respiratory organ dysfunction was most common (n=7,768, 43.92%), and cardiovascular system (n=2,298, 12.99%) (Table 3). Overall, 6,893 (38.97%) patients had sepsis during their ICU stay.
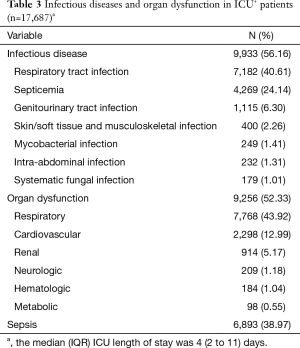
Full table
Risk factors for 1-year mortality among ICU+ patients
A multivariate analysis indicated that independent risk factors for mortality for ICU+ lung cancer patients were age (≥75 years) [adjusted hazard ratio (AHR), 1.22; 95% CI, 1.16–1.29], male sex (AHR, 1.18; 95% CI, 1.13–1.23), recent radiotherapy (AHR, 1.09; 95% CI, 1.04–1.15), infectious diseases (AHR, 1.23; 95% CI, 1.17–1.29), organ dysfunction (AHR, 1.57; 95% CI, 1.50–1.65), and lower-level hospitals (Table 4). In contrast, ICU+ patients treated with recent lung cancer surgery (AHR, 0.19; 95% CI, 0.17–0.21), chemotherapy (AHR, 0.84; 95% CI, 0.80–0.88), or targeted therapy (AHR, 0.32; 95% CI, 0.30–0.35) were associated with lower mortality (Table 4).
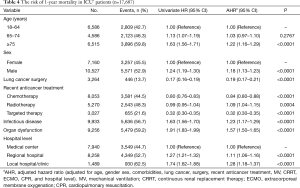
Full table
Discussion
This is the first national population-based study to specifically investigate the outcomes of ICU+ lung cancer patients. We have several significant findings. First, in almost all subgroup analyses, the overall 1-year mortality rate was significantly higher, and the time to 1-year mortality was significant shorter, for ICU+ patients. Survival analysis showed that patients admitted to ICU had lower probability of 1-year survival rate than patients without ICU admission. The differences regarding survival rate become evident just soon after ICU admission. The poor impact of ICU admission on the 1-year mortality remained significant in almost all of subgroup analysis.
Second, we found that about 40% of ICU+ lung cancer patients had sepsis, and more than 50% had an organ dysfunction: respiratory tract infections and respiratory organ dysfunction were most common. This finding is consistent the previous study (9) of 143 lung cancer which showed that the main reasons for ICU admission were sepsis (44%) and acute respiratory failure (31%). This is reasonable (9,17,18). Overall, this kind of information will help intensivists improve treatment for ICU+ lung cancer patients.
Third, we identified several factors that indicate a poor prognosis: age, male sex, recent radiotherapy, infectious diseases, organ dysfunction, and low hospital level, some of have already been reported (9,19). Chang et al. (19) enrolling 143 patients with lung cancer and pneumonia-induced respiratory failure in a medical intensive care unit showed that history of radiotherapy (OR =2.80; 95% CI, 1.15–6.78) was associated with increased mortality. Soares et al. (9) found that number of organ failures (OR =1.96; 95% CI, 1.38–2.79) was associated with increased mortality. Previous study (20) has showed that hospital volume can affect the outcome of cancer patients undergoing anti-cancer treatment. In this study, we found that hospital level was independently associated with mortality. Even sicker patients referred to medical centers had better survival rates than did patients in regional and local hospitals. The finding is consistent with previous evidence (20) that larger hospital volume or specialty focus is associated with the better outcome of cancer patients.
Finally, we found that recent surgery, chemotherapy, and targeted therapy all lowered the mortality risk, which is consistent with other claims (6). The similar finding was noted in previous study (6) that anticancer therapy in the ICU might provide better short-term ICU survival for treatment-naïve, critically ill lung cancer patients. It is possible that the patients who were chosen to receive anti-cancer therapy may have better performance status and could tolerate the treatment-related complication than the patients who physicians cannot recommend anti-cancer therapy such as chemotherapy or surgery due to their poorer performance status. Taking all these findings together suggest that, although anticancer treatment causes complications, it will positively affect survival for some patients. However, additional studies are required to determine which specific populations will benefit from which anticancer treatments.
This study has several strengths. We studied a large sample from Taiwan’s general population, did a long-term follow-up, and included the full range of hospital levels from local clinics to medical centers. These strengths should make our findings generalizable to most Taiwanese.
However, a retrospective insurance claims database analysis also has several inherent limitations. First, the NHIRD information is provided using ICD-9 coding, not actual medical records. However, this limitation might not affect our findings, because lung cancer in this study was based on a catastrophic illness card that can be obtained only after the diagnosis is confirmed by a biopsy and histological testing. Second, we chose only first-episode ICU+ patients admitted more than once during the study period, but patients with lung cancer might be readmitted in short time-spans for anticancer treatment-related complications. Third, the criteria for ICU admission vary by hospital, especially for hospitals at different levels. This might increase heterogeneity into the study cohort. Finally, the NHIRD does not contain data about specific histologic types or stages of cancer, specific cause of death, Eastern Cooperative Oncology Group (ECOG) performance status, and we did not assess the effect of different anticancer treatments and the number of cancer-related mortality.
Conclusions
ICU admission for lung cancer patients is associated with higher mortality. Several risk factors of mortality after ICU admission identified in this study can help physicians provide personalized and better-informed decisions for managing lung cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board (IRB No: 10411-E01) of Chi Mei Medical Center. Because the data used in this study are de-identified, and have been released to the public for research, the IRB waived informed consent.
References
- Sugimura H, Yang P. Long-term survivorship in lung cancer: a review. Chest 2006;129:1088-97. [Crossref] [PubMed]
- Heuvers ME, Hegmans JP, Stricker BH, et al. Improving lung cancer survival; time to move on. BMC Pulm Med 2012;12:77. [Crossref] [PubMed]
- Vachani A, Sequist LV, Spira A. AJRCCM: 100-Year Anniversary. The Shifting Landscape for Lung Cancer: Past, Present, and Future. Am J Respir Crit Care Med 2017;195:1150-60. [Crossref] [PubMed]
- Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271-89. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Chen YF, Lin JW, Ho CC, et al. Outcomes of cancer therapy administered to treatment-naive lung cancer patients in the intensive care unit. J Cancer 2017;8:1995-2003. [Crossref] [PubMed]
- Lin YC, Tsai YH, Huang CC, et al. Outcome of lung cancer patients with acute respiratory failure requiring mechanical ventilation. Respir Med 2004;98:43-51. [Crossref] [PubMed]
- Reichner CA, Thompson JA, O'Brien S, et al. Outcome and code status of lung cancer patients admitted to the medical ICU. Chest 2006;130:719-23. [Crossref] [PubMed]
- Soares M, Darmon M, Salluh JIF, et al. Prognosis of lung cancer patients with life-threatening complications. Chest 2007;131:840-6. [Crossref] [PubMed]
- Roques S, Parrot A, Lavole A, et al. Six-month prognosis of patients with lung cancer admitted to the intensive care unit. Intensive Care Med 2009;35:2044-50. [Crossref] [PubMed]
- Adam AK, Soubani AO. Outcome and prognostic factors of lung cancer patients admitted to the medical intensive care unit. Eur Respir J 2008;31:47-53. [Crossref] [PubMed]
- Müller AM, Gazzana MB, Silva DR. Outcomes for patients with lung cancer admitted to intensive care units. Rev Bras Ter Intensiva 2013;25:12-6. [PubMed]
- Boussat S, El'rini T, Dubiez A, et al. Predictive factors of death in primary lung cancer patients on admission to the intensive care unit. Intensive Care Med 2000;26:1811-6. [Crossref] [PubMed]
- Toffart AC, Minet C, Raynard B, et al. Use of intensive care in patients with nonresectable lung cancer. Chest 2011;139:101-8. [Crossref] [PubMed]
- Kim YJ, Kim MJ, Cho YJ, et al. Who should be admitted to the intensive care unit? The outcome of intensive care unit admission in stage IIIB-IV lung cancer patients. Med Oncol 2014;31:847. [Crossref] [PubMed]
- Wen CP, Tsai SP, Chung WS. A 10-year experience with universal health insurance in Taiwan: measuring changes in health and health disparity. Ann Intern Med 2008;148:258-67. [Crossref] [PubMed]
- Rosolem MM, Rabello LS, Lisboa T, et al. Critically ill patients with cancer and sepsis: clinical course and prognostic factors. J Crit Care 2012;27:301-7. [Crossref] [PubMed]
- Torres VB, Azevedo LC, Silva UV, et al. Sepsis-Associated Outcomes in Critically Ill Patients with Malignancies. Ann Am Thorac Soc 2015;12:1185-92. [PubMed]
- Chang Y, Huh JW, Hong SB, et al. Outcomes and prognostic factors of patients with lung cancer and pneumonia-induced respiratory failure in a medical intensive care unit: a single-center study. J Crit Care 2014;29:414-9. [Crossref] [PubMed]
- Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: importance in quality of cancer care. J Clin Oncol 2000;18:2327-40. [Crossref] [PubMed]