Changes of pleural pressure after thoracic surgery
Introduction
Pleural pressure (Ppl) is the pressure in the thin space between the visceral and parietal pleura. During quiet breathing, Ppl is normally slightly subatmospheric, between −3 and −5 cmH2O (1). Ppl is critical to maintain lung expansion during respiration and to explain the pathophysiology of respiratory abnormalities, such as pneumothorax, ventilator-induced lung injury, and acute respiratory distress syndrome (2,3). However, the actual Ppl values in various clinical conditions are not well reported because direct measurement of Ppl is inevitably invasive.
Since most postoperative patients in a thoracic department have a thoracic catheter through which the Ppl can be easily checked without any additional risk, several studies on the direct measurement of Ppl have been conducted in thoracic patients (4-10). However, previous reports had limitations in terms of understanding the actual physiologic changes of Ppl after various types of pulmonary resection; the measured Ppl was not the actual Ppl after pulmonary resection because air leakage was present or thoracic suction was applied during measurement (4-6), it is difficult to know the wave of Ppl in each single respiration because Ppl was reported as a mean value over a certain period of time (4,7), they used commercial devices no longer available (4,7,8), Ppl was measured during drainage of pleural effusion (9,10), and the purpose of research is to simply compare the validity of Ppl measuring instruments (9). Furthermore, no study that measured Ppl distinguished between routine and forced breathing after pulmonary resection (4-10). We thought that it was important to determine the actual Ppl during deep inspiration after lobectomy because the negative pressure produced by strong skeletal accessory inspiratory muscles might be too high in patients with reduced lung volume after surgery, which could provoke clinical problems such as delayed air leakage or pneumomediastinum.
We manufactured a digital manometer to measure the actual Ppl after thoracic surgery, and compared the Ppl according to the type of pulmonary resection during quiet and forced breathing.
Methods
Patients characteristics
In this single-center prospective observational study, we measured the Ppl of patients who underwent various types of thoracic surgery from August to September, 2017. Patients were excluded from this study according to the following criteria: confirmed excessive pleural adhesion or pleural thickening during operation, postoperative pneumonia or significant atelectasis, or any reason that could restrict movement of the chest wall or diaphragm such as phrenic nerve palsy or conversion to thoracotomy. All patients underwent pulmonary resection by board-certified thoracic surgeons (S Choi, DK Kim, and GD Lee) and were divided into two groups, the lobectomy group and the minimal/no-resection group, to compare the effect of the extent of pulmonary resection. In all patients we used 3 or 4 ports VATS technique. In lobectomy group, the working window was approximately 4 cm, and other 2 or 3 ports were 5 or 12 mm ports. In minimal/no-resection group, all the ports were 5 or 12 mm ports. All the patients routinely used intravenous patient-controlled analgesia. One chest tube was inserted in each patient. The Ppl was measured the day before chest tube removal. We routinely remove chest tube on 3rd or 4th POD in lobectomy group, and 2nd day in wedge resection group. During measurements, the distal part of the chest tube was clamped to guarantee that the pressure inside the chest tube was same as the intra-thoracic pressure. This study was approved by the institutional review board of Asan Medical Center in Seoul, South Korea (IRB No. 2017-0386). We are well acknowledged that to obtain appropriate informed consent for publication is our responsibility, and we obtained the written informed consent from the patients when we checked the Ppl.
Digital manometer
Researchers of the Department of Biomedical Engineering (D Lim, SK Joo) have manufactured a digital manometer as shown in Figure 1. The device has a liquid-crystal display (LCD, QY-164A, QY) and two buttons, one for quiet-mode measurement and another for forced-mode measurement. On the right side of the top of the device, there is one port to be connected to a pressure measuring tube. Figure 1B shows the main printed circuit board. The device is controlled by a microcontroller (Atmega16, Atmel, Microchip Technology Inc., AZ, USA), which controls the display, receives converted pressure data, and sends it to a Bluetooth module (BoT-CLE110D, Chipsen, Gyeonggi, Korea) for wireless communication with other devices. The gauge pressure of the pleura is measured with a gauge pressure sensor (MPXV7007GP, NXP USA Inc., TX, USA), which can measure gauge pressure from −10 to 10 kPa (−100 to 100 cmH2O). Analog output of the sensor is converted to a digital signal by an analog-to-digital converter (ADC, MCP3204, Microchip Technology Inc., AZ, USA) with a resolution of 12 bits and a sampling rate of 50 Hz. The sampling rate of 50 Hz is fast enough since the normal respiration rate is about 15 breaths/min. The converted signal is then sent to the microcontroller. A 9-volt battery is used to supply power to the device. The size of the developed device is 147 mm (width) × 86 mm (length) × 40 mm (height). Within the measurement, a real-time pressure value is shown on the LCD. After the measurement is done, the maximum, minimum, and average pressure values during the measurement are displayed on the LCD. If the device is connected to any devices via Bluetooth, the real-time pressure data is also transmitted to the connected computer, phone, or tablet computer as shown Figure 1C. Measurements are taken 50 times per second at a resolution of 0.02 cmH2O and these values are recorded in an Excel (Microsoft, WA, USA) spreadsheet. By converting the values in a graph, the Ppl can be expressed as a wave form.

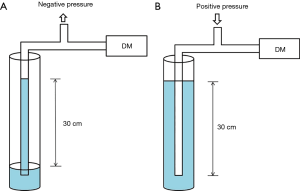
The accuracy of the pressure measuring device was confirmed by comparing it to that of the conventional analogue method using a water column. To check the negative pressure value, we aspirated water from the bottom of the water column up to the 30 cm line with the other end connected to the digital manometer. Similarly, the positive value of the digital manometer was measured while pushing air through the 30 cm line of the water-filled column. The positive and negative pressure values were exactly same as measured by both the digital manometer and conventional method (Figure 2).
Ppl measuring
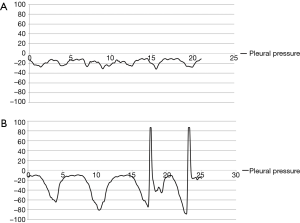
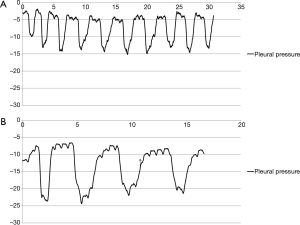
Ppl was measured by direct insertion of a 23-gauge needle connected to the manometer into the chest tube (usually 28 Fr). The tubes were carefully examined to remove any effusion from the lumen before measurement, and the distal part of chest tube from the point where the needle was inserted was clamped during Ppl measurement to confirm that the pressure of the lumen was the same as the intra-thoracic pressure. All chest tubes were located in the posterior apex to exclude the effect of the weight of the lung, and the patients were seated upright on a bed during the measurement. With the needle of the manometer in place while checking the Ppl, the patients were asked to perform periods of quiet and forced breathing for about 30 seconds each, coughing several times after forced breathing. All pressure values were converted to a graph to confirm that the Ppl wave was obtained correctly (Figure 3). If patients could not obey the instructions correctly or if there were the outliers or erroneously measured values during the measurement period, for example, coughing during quiet respiration or zeroing data included in the forced pressure, we cut these periods of erroneous data from the analysis. Ppl was expressed as cmH2O.
Statistical analysis
We used the Student’s t-test to compare the mean value of Ppl between groups. A P value smaller than 0.05 was considered statistically significant. The statistical analysis was performed using SPSS version 22.0 (SPSS INC., NY, USA).
Results
A total of 23 patients were enrolled in the lobectomy group, and the diagnosis of these patients was mainly non-small cell lung cancer with the exception of 1 patient who had pulmonary metastasis from renal cell cancer. The resected lobes were 9 RUL, 2 RML, 6 RLL, 3 LUL and 3 LLL, each (Table 1). The diagnoses of the 20 patients in the minimal/no-resection group were diverse: interstitial lung disease (ILD) in 5, wedge resection for metastatic lung cancer in 5, wedge resection for non-small cell lung cancer in 3, videoscope-assisted mediastinal lymph node biopsy due to lymphoma in 3, wedge resection for pneumothorax in 2, extralobar pulmonary sequestration in 1, and excision of pericardial cyst in 1 (Table 2). The mean age was significantly older in the lobectomy group than the minimal/no-resection group; however, other characteristics, including smoking history, gender difference, body-mass index and pulmonary function, were not statistically different between the two groups (Table 3).
Full table
Full table

Full table
When the patients breathed normally, the mean Ppl was significantly lower in lobectomy group compared to the minimal/no-resection group (−11.2 vs. −8.3 cmH2O, P=0.026) (Table 4). During forced respiration, all of the Ppl values at end inspiration, end expiration, and for the mean pressure were significantly lower in lobectomy group (−44.0 vs. −29.8 cmH2O at end inspiration, −4.2 vs. −0.1 cmH2O at end expiration, and −18.9 and −12.7 cmH2O for mean Ppl, each). The maximal pressures during coughing were not different between the two groups (38.4 vs. 34.4 cmH2O, P=0.687).

Full table
Discussion
In this study, we measured the actual Ppl directly through indwelling chest tubes in postoperative thoracic patients. To our knowledge, this is the first report on how much the Ppl can decrease during deep inspiration after lobectomy in an actual clinical field. As expected, the Ppl was lower in the lobectomy group compared to the minimal/no-resection group. The difference in Ppl between the two groups when patients breathed normally was marginally significant; however, the values were significantly different during forced ventilation, especially the end inspiration and mean pressure values. There may be several reasons why the pressure difference was marginally different during quiet respiration. First, during quiet ventilation, the strong accessory respiration muscles such as sternocleidomastoid and scalene muscles are not activated, so the power to make a negative Ppl is limited. Second, ILD patients with reduced lung volume were included in the minimal resection group. For example, the diagnosis of a patient who measured −87.2 cmH2O at deep inspiration was ILD. This patient had a wedge resection in the left upper and lower lobes while his left lower lobe was fibrotic and the volume was reduced just like in the lobectomy patients. The peak pressure at coughing was almost same in both groups. We think the reason is that the pressure during coughing is largely dependent on the power of the respiration muscles rather than the lung volume, because coughing occurs when there’s an isovolumic contraction of the chest wall against a closed vocal cord. The observations that the negative pressure could go down to more than negative 80 cmH2O during deep inspiration and that the mean difference of Ppl during forced respiration was more than 80 cmH2O in lobectomy patients have significant clinical meaning in the postoperative period. An inspirometer or pulmonary rehabilitation is usually recommended after an operation to prevent postoperative pneumonia or to preserve pulmonary function, though the role of pulmonary rehabilitation after thoracic surgery remains unclear (11,12). We do not think this level of negative pressure or differential pressure is physiologically tolerable for the patients who underwent lobectomy, especially in the immediate postoperative period. Excessive negative pressure during immediate postoperative period is thought to be related with prolonged air leakage or longer chest tube indwelling.
The direct measurement of Ppl through chest tubes allowed us to expand our understanding of respiration physiology. Traditionally the Ppl is explained to be determined by the lung condition; for example, in emphysematous patients, the Ppl is relatively less negative compared to that of fibrotic lung (1,2). However, in conducting this study, we determined that the thoracic cage volume and the condition of the respiratory muscles are also important factors in determining Ppl. Although not included in this study, Ppl varied dramatically according to the ‘empty’ thorax volume in a post-pneumonectomy patient (Figure 4). Similarly, reduced thoracic volume after lobectomy can induce more negative pressure during inspiration. The process is completely opposite with that of emphysema patients, whose thoraxes are already fully expanded. Thoracic volume acts as a preload in respiration mechanics. We think this result could be meaningful in understanding the pathophysiology of acute respiratory distress syndrome, pneumonia, or lobar atelectasis patients, whose lung volume is decreased reversibly.
There are limitations in this study. First, it does not reflect the Ppl of the lower thorax or of a patient lying down, because the Ppl was measured through chest tubes located in the apex and in sitting position only. Second, the mean patient age was different between the two groups, because a lobectomy was performed mainly in lung cancer patients, whereas wedge resection was performed for a diagnosis of interstitial lung disease, single pulmonary nodule, or ground-glass nodules. To reduce the effect of lung conditions of patients, we included patients with normal pulmonary function only. Third, many other factors influence the Ppl such as postoperative pain, patient compliance, obesity, or the physical activity of the patients. To control these factors, we included videoscope-assisted patients only, checked the Ppl before chest tube removal, and evaluated the cooperation of the patients by checking the Ppl wave when analyzing the data. The effect of abdominal pressure in obese patients could be reduced by checking the Ppl in an upright position.
Conclusions
We report the actual intrapleural pressure changes according to respiration and types of surgery using digital manometer through chest tubes. In lobectomy patients, the intrapleural pressure was highly negative compared to that of the minimal/no-resection group, especially during forced respiration.
Acknowledgements
Funding: This work was supported by Asan Institute for Life Sciences, Asan Medical Center, Seoul, Republic of Korea [2017-597].
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review board of Asan Medical Center in Seoul, South Korea (IRB No. 2017-0386). We obtained the written informed consent from the patients when we checked the pleural pressure.
References
- Levitzky MG. Mechanics of Breathing. In: Levitzky MG. Pulmonary Physiology. 8th ed. McGraw-Hill companies; 2013. p 12-57.
- Miserocchi G, Beretta E, Rivolta I. Respiratory mechanics and fluid dynamics after lung resection surgery. Thorac Surg Clin 2010;20:345-57. [Crossref] [PubMed]
- Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013;369:2126-36. [Crossref] [PubMed]
- Varela G, Brunelli A, Jime’nez MF, et al. Chest drainage suction decreases differential pleural pressure after upper lobectomy and has no effect after lower lobectomy. Eur J Cardiothorac Surg 2010;37:531-4. [Crossref] [PubMed]
- Brunelli A, Cassivi SD, Fibla J, et al. Pleural pressure immediately after pulmonary lobectomy: Single versus double chest tubes for suction. J Thorac Cardiovasc Surg 2010;140:e52-3. [Crossref] [PubMed]
- Brunelli A, Cassivi SD, Salati M, et al. Digital measurements of air leak flow and intrapleural pressures in the immediate postoperative period predict risk of prolonged air leak after pulmonary lobectomy. Eur J Cardiothorac Surg 2011;39:584-8. [Crossref] [PubMed]
- Refai M, Brunelli A, Varela G, et al. The values of intrapleural pressure before the removal of chest tube in non-complicated pulmonary lobectomies. Eur J Cardiothorac Surg 2012;41:831-3. [Crossref] [PubMed]
- Cerfolio RJ, Varela G, Brunelli A. Digital and Smart Chest Drainage Systems to Monitor Air Leaks: The Birth of a New Era? Thorac Surg Clin 2010;20:413-20. [Crossref] [PubMed]
- Lee HJ, Yarmus L, Kidd D, et al. Comparison of Pleural Pressure Measuring Instruments. Chest 2014;146:1007-12. [Crossref] [PubMed]
- Boshuizen RC, Sinaasappel M, Vincent AD, et al. Pleural Pressure Swing and Lung Expansion After Malignant Pleural Effusion Drainage The Benefits of High-Temporal Resolution Pleural Manometry. J J Bronchology Interv Pulmonol 2013;20:200-5. [Crossref] [PubMed]
- Rivas-Perez H, Nana-Sinkam P. Integrating pulmonary rehabilitation into the multidisciplinary management of lung cancer: A review. Respir Med 2015;109:437-42. [Crossref] [PubMed]
- Cesario A, Ferri L, Galetta D, et al. Post-operative respiratory rehabilitation after lung resection for non-small cell lung cancer. Lung Cancer 2007;57:175-80. [Crossref] [PubMed]


