Lung function impairment is not associated with the severity of acute coronary syndrome but is associated with a shorter stay in the coronary care unit
Introduction
Lung function impairment (LFI) is common in the general population and is often undiagnosed (1). Globally, the estimated prevalence of diseases associated with chronic airflow obstruction is over 13% in the adult population (2). These diseases are primarily chronic obstructive pulmonary disease (COPD) and asthma (3). The prevalence of diseases associated with a degree of restrictive defect, either due to obesity, interstitial lung disease, or abnormalities of the thoracic cage, is approximately 6% (3).
Although the severity of LFI itself confers a poor prognosis due to the appearance of pulmonary complications in patients with respiratory disease (4), both the forced expiratory volume in one second (FEV1) and the forced vital capacity (FVC) are well established as predictors of mortality and/or respiratory complications in the general population (5). This poor prognosis is mainly due to the development of cardiovascular events, regardless of other factors such as smoking exposure (6-8). Although the specific mechanisms of this association are not fully understood, it has been postulated that systemic inflammation, an increased sympathetic tone, and arterial stiffness in patients with LFI may have a specific role in the increased cardiovascular risk (9,10).
Based on this evidence, an observational study was conducted to assess the impact of LFI on the severity and short-term prognosis of acute coronary syndrome (ACS). The aim of this study was to compare the ejection fraction, Killip class, number of diseased vessels, peak troponin levels, length of hospital admission, complications, and mortality in a cohort of subjects with ACS with and without previously known LFI.
Methods
Subjects
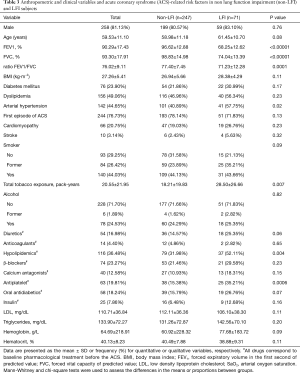
This was an ancillary study of the ISAACC trial [NCT01335087, continuous positive airway pressure (CPAP) in subjects with ACS and obstructive sleep apnea (OSA)], which is a multicenter, open-label, parallel, prospective, randomized, controlled trial that evaluates the effect of CPAP treatment on the incidence of new cardiovascular events in subjects with an episode of ACS and OSA (11). In the present study, subjects who were admitted to coronary care units (CCUs) and cardiology wards at thirteen teaching hospitals in Spain due to ACS (men and women ≥18 years old) and in whom spirometry was performed were included. Subjects were recruited from April 2011 to September 2014. Included subjects underwent respiratory polygraphy (RP) during the first 48–72 h of admission. Subjects with an apnea-hypopnea index (AHI) >15 events per·hour were randomized to conservative or CPAP treatment.
In the current study, the short-term prognosis and severity of ACS in subjects with and without LFI who were included in the conservative arm and in the control group were compared and, additionally, the interaction with OSA was evaluated.
ACS was defined as the acute presentation of coronary disease with or without ST elevation infarction, unstable angina, or type 1 myocardial infarction (MI) (12). The exclusion criteria included the following: previous treatment with CPAP; psychophysical inability to complete questionnaires; the presence of any previously diagnosed sleep disorder; subjects with >50% central apnoeas or the presence of Cheyne-Stokes respiration; daytime sleepiness [Epworth Sleep Scale (ESS) >10]; subjects with chronic disease (including COPD with severe airflow limitation defined by a FEV1 <50% of predicted) (13), neoplasms, renal insufficiency with a glomerular filtration rate (GFR) <15 mL/min/1.73 m2, chronic depression, or any other limiting chronic disease); a medical history that could interfere with the study objectives; any conditions, e.g., cardiovascular, that reduced life expectancy to <1 year; and subjects in cardiogenic shock.
Variables such as alcohol intake and smoking were evaluated. The accumulative tobacco exposure (packs-year) and the smoking status were recorded following definitions of the Centers for Disease Control and Prevention (14).
The ethics committee of each participating center approved the study (approval number in the coordinator center: 2010-852), and subjects provided written informed consent.
Procedures
Spirometry without a bronchodilator test was carried out in each university hospital by experienced respiratory technicians one to three months after the ACS, following Spanish guidelines (15), which are in accordance with the quality and standardization criteria proposed by the ATS/ERS task force (16). LFI was defined as a FVC and/or FEV1 <80% of the predicted value (17), and standardization was carried out using validated equations from a reference European population, which are also recommended by the national consensus (15,18). The presence of obstructive LFI was considered when the FEV1/FVC ratio was <0.70 (13,17,19). For statistical purposes and subsequent analysis, subjects who did not meet the criteria for LFI and who also had FVC >100% of predicted were defined as “supra-normal” (20). At the time of examination, none of the subjects had a previous history of significant pulmonary disease.
Echocardiographic evaluation and Killip classification were routinely performed during patient admission. The Killip classification focuses on physical examination and the development of heart failure to predict risk. The classification considers four classes (I to IV). Class I indicates no evidence of heart failure, and class IV represents cardiogenic shock (21). The measurement of the left ventricular ejection fraction (LVEF) was performed by the modified Simpson’s rule (22). During the hospitalization period were evaluated the severity of ACS (LVEF, Killip class, number of affected vessels, number of stents implanted, and peak troponin level) and the short-term prognosis defined by the outcome during the hospitalization period: length of stay in the CCU, length of hospitalization, complications (cardiogenic shock, heart failure, cardiac rupture, acute severe mitral regurgitation, sustained ventricular arrhythmias or ventricular fibrillation, and cardio-respiratory arrest) and mortality. The cardiologists who evaluated the ACS severity were blinded to the OSA versus control status.
The diagnosis of OSA was based on the results of the sleep test, which is in accordance with the guidelines of the Spanish national consensus on the apnea-hypopnea syndrome (23). All participant centers used the same polygraph model (Embletta; ResMed, Australia) for the diagnosis of OSA. Oronasal flow, thoracoabdominal movements, an electrocardiogram (ECG), and pulse oximetry were recorded. Apnea was defined as an absence of airflow lasting ≥10 s. Hypopnea was defined as a reduction in airflow lasting ≥10 s that was associated with oxygen desaturation. Oxygen desaturation was defined as a decrease in oxygen saturation (SaO2) >4%. RP studies were performed without supplemental oxygen. The AIH was defined as the number of episodes of apnea and hypopnea per hour of recording. The degree of self-reported sleepiness/drowsiness was analyzed by the Spanish version of the ESS test (24).
Statistical analyses
An anonymized database was generated, and only the coordinating center (Hospital Univ. Arnau de Vilanova and Santa Maria, IRBLLeida, Lleida, Spain) had access to it. Firstly, the means ± SD or frequencies (%) were calculated for quantitative and qualitative variables, respectively, to evaluate the difference between LFI and non-LFI subjects with respect to clinical and anthropometric variables and LFI- and ACS-related factors. Statistical significance was assessed with the Mann-Whitney or chi-square test as appropriate. Second, the association of LFI with variables related to ACS severity and short-term prognosis was assessed with Mann-Whitney or chi-square tests and linear or logistic regression models. The exact binomial test was used instead of the chi-square test to assess the observed number of cardiovascular complications during hospitalization due to their low frequency. Moreover, multiple regression models were fitted to adjust for potentially confounding variables, including hypertension, age, and sex. Third, the interaction of OSA with LFI with respect to the length of stay in the CCU or cardiology ward was evaluated with linear regression models by considering both the main effects of OSA and LFI as well as their interaction. Multiple regression models that included the effect of potentially confounding variables were utilized as well. The significance interaction was statistically assessed with the F-test to compare the goodness of fit of the models with and without the interaction terms. All analyses were performed using the R statistical package (R Core Team: www.R-project.org, Accessed February 5, 2014), and the threshold for significance was set at 0.05.
Sample size
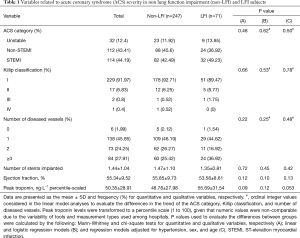
This was an observational study, and therefore, a post-hoc study was conducted to determine the statistical power to detect differences between the two groups. The sample population included 71 subjects with LFI and 247 without LFI, which provided a statistical power of 80% to detect a minimum increase in mean troponin levels in the LFI subjects of 81.27%, a minimum decrease in the mean ejection fraction (percentile-scaled) of 21.77%, and a minimum decrease of 20.45% in the length of stay in the CCU. These calculations were performed based on the mean values and standard deviations reported in Tables 1,2 and using a two-sample t-test to statistically assess the differences with a 5% type I error (α=0.05).
Full table

Full table
Results
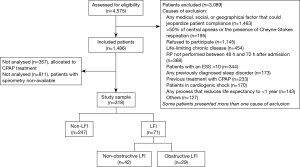
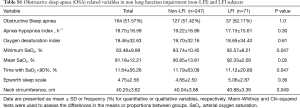
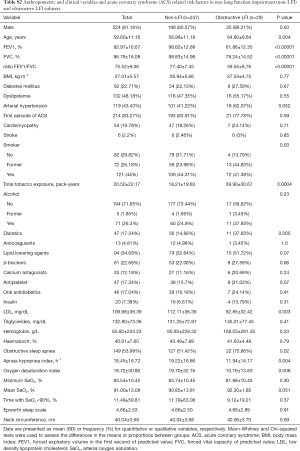
We recruited 318 subjects, including 71 with LFI (22.33%) and 247 without LFI (77.67%) (Figure 1). No differences were found in sex, age, or body mass index (BMI) between the groups, but the proportion of hypertensive subjects was significantly higher in the LFI group and, as expected, these subjects had a higher tobacco exposure (Table 3). The proportion of subjects treated with lipid-lowering agents, and antiplatelet drugs was higher in the LFI group (Table 3). With respect to OSA-related variables, some significant differences between groups were found. There was a lower minimum SaO2 and mean SaO2 and a higher time with SaO2 <90% and neck circumference in LFI subjects than in non-LFI subjects (Table S1), although these differences were not considered clinically relevant. A specific analysis performed to compare non-LFI with obstructive subtype LFI subjects (247 and 29 subjects, respectively) showed similar characteristics (Table S2), with the exception of age (obstructive LFI subjects were older) and the use of diuretics (a greater proportion of obstructive LFI subjects used diuretics).
Full table
Full table
Full table
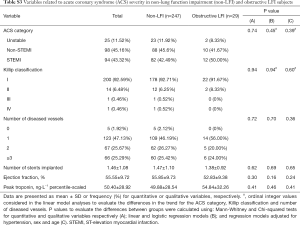
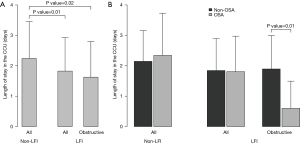
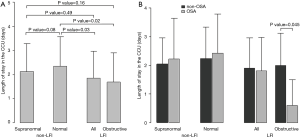
With respect to ACS severity, no association with LFI was found for any of the variables analyzed (Table 1), even when considering obstructive LFI (Table S3). However, with respect to the short-term prognosis of ACS, the length of stay in the CCU was significantly shorter for the LFI subjects than the non-LFI subjects (Table 2, Figure 2A). In addition, obstructive LFI subjects had an even shorter length of stay in the CCU (Table S4, Figure 2A).
Full table
Full table
Full table
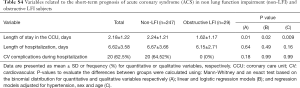
Nevertheless, no significant differences between groups were found with respect to the length of hospitalization and the number of cardiovascular complications during hospitalization.
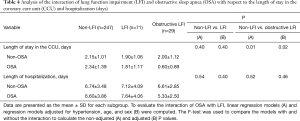
A further analysis of the differences between non-LFI and LFI subjects with respect to length of stay in the CCU revealed a significant interaction of LFI with OSA only in obstructive LFI subjects (Table 4). Indeed, subjects with both obstructive LFI and OSA had the shortest length of stay in the CCU (Table 4, Figure 2B) compared with any other group. Furthermore, no differences were found between subjects with “supra-normal” FVC values (FVC >100% of predicted) and subjects with a FVC between 80 and 100% of predicted (99 vs. 148 subjects in the non-LFI group), confirming the finding of a shorter length of stay for obstructive LFI subjects (Table S5, Figure S1).

Full table
Discussion
The results of this observational study suggest that LFI does not have a significant influence on the severity of ACS (determined by the peak plasma troponin level, decreased ejection fraction, and number of affected vessels) in this cohort of subjects and is not related to a worse short-term prognosis. In contrast, subjects with LFI had a shorter length of stay in the CCU (which is considered a measurement of short-term prognosis), and this relationship was more evident in subjects with obstructive LFI who also had OSA.
Data from population-based studies suggest that LFI measured by a low FVC and/or FEV1 is associated with an increased incidence of cardiovascular events (6,25-28). In fact, it has been established that there is a 1.5- to 5-fold increased risk of coronary artery disease in subjects with chronic airflow limitation (29). Oxidative stress, systemic inflammation, endothelial dysfunction, loss of extracellular matrix components such as elastin (mainly in emphysema cases), a hypercoagulable state, and an increase in sympathetic tone have been proposed as the underlying mechanisms responsible for the increase in cardiovascular events in subjects with COPD (30,31). Additionally, Sabater-Lleal et al. (32) demonstrated an association between common variations in certain genes related to abnormal lung physiology with those associated with powerful markers of cardiovascular disease, supporting a hypothesis of a strong relationship at all levels. However, only a few studies have directly measured the impact of reduced lung function on short-term prognosis and fatality rate from cardiovascular events. In a Swedish population-based cohort, Engström et al. (33) showed that the probability of dying during the first 24 h after an acute coronary event were almost doubled in subjects with LFI compared with those with normal lung function. Supporting these findings, there is evidence that changes in pulmonary function are associated with an increased mortality due to ventricular arrhythmias, which are a common cause of short-term fatality after an acute coronary event (34,35). Our results do not confirm the association between LFI and ACS severity, but they actually show a paradoxical effect in relation to one of the markers of short-term prognosis; specifically, subjects with LFI had a statistically significantly shorter stay in the CCU, and this association was stronger in subjects with obstructive LFI. Interestingly, when data from subjects with other subclinical respiratory comorbidities, such as OSA, were taken into account, the length of stay in the CCU of subjects with obstructive LFI was even lower. Although we do not provide any evidence of the reproducibility of this finding in other clinical settings, the magnitude of this difference in the length of stay in the CCU between groups in our study appears clinically relevant. To justify these findings that LFI could confer a certain cardioprotective role after an ACS, the hypothesis of ischemic preconditioning may be used in the same manner that it has in the past regarding patients with OSA. This hypothesis posits that certain adaptive mechanisms may be present after a sustained mild or moderate exposure to chronic hypoxia, which could also occur in subjects with subclinical and chronic LFI (36). Similarly, a phenomenon termed “the smoker’s paradox” has been postulated, namely, smokers have a higher incidence of acute MI but improved survival after reperfusion. There are several hypotheses to support this fact, but it has been observed in previous studies that less extensive angiographic coronary disease is found in smokers than in their non-smoking counterparts (37). In our cohort of subjects, there was significantly higher tobacco exposure among those with LFI and ACS compared with that among the non-LFI subjects, but there were no significant differences in the global smoking status.
The strengths of our study include its multicenter design with a relatively large number of subjects. However, clear potential limitations are evident. Firstly, due to the design of the ISAACC Study, subjects with more severe ACS, and therefore with a worse prognosis at the time of presentation, were excluded, as were those with a history of known severe respiratory disease; therefore, most subjects had only mild or moderate LFI. These are the main reasons why it is not possible to generalize these results to other subgroups of subjects. Second, spirometry was performed at least one month after the ACS, when certain changes in lifestyle (e.g., weight, smoking status) or medical treatment could have caused improvement or worsening of the spirometry values compared with lung function measurements prior to the event. However, the fact that we were unable to identify relevant differences in either the anthropometric features or in the degree of drug exposure between LFI and non-LFI subjects strengthens the results of this study. Third, the fact of not having a specific protocol within the ISAACC study for the performance of spirometry (e.g., single and standardized equipment) is a potential limitation that must be recognized when interpreting the results. However, all participating hospitals are academic centers with specific pulmonary function laboratories where spirometry measurements are well standardized, so the results are fully valid in terms of quality of the spirometric values. Finally, the most relevant potential limitation has to do with the definition used to establish the presence of LFI, “supra-normal” values and airflow limitation from fixed cut-off points and not using the lower or upper limit of normality (LIN or ULN) or z-scores, which are recommended by current guidelines in the field of epidemiological research and also in the clinical setting due to the risk of misclassification, especially in young subjects and in the elderly (38). Unfortunately, in our study these values were not available directly from each recruitment center and calculation, although possible a posteriori could be a source of error. In any case, we believe that due to the characteristics of the included subjects (e.g., mean age) it is feasible to consider that the cut-off points used do not overestimate or underestimate the presence of LFI or airflow limitation in a significant way.
Conclusions
The results of this observational study suggest that there is a clinical phenotype of subjects with ACS in whom the presence of LFI and other respiratory comorbidities (e.g., OSA) may be associated with a better short-term prognosis (determined by a shorter stay in the CCU) that is independent of the severity of the underlying event.
Acknowledgements
Funding: This study was supported by: ResMed Ltd. (Australia), Fondo de Investigación Sanitaria (PI10/02763 and PI10/02745), the Spanish Respiratory Society (SEPAR), the Catalonian Cardiology Society, Esteve-Teijin (Spain), Oxigen Salud (Spain), and ALLER.
Footnote
Conflicts of Interest: F Barbé received a research grant from: ResMed Inc., Australia, a company that develops products related to sleep apnea, the Health Research Fund, Spanish Ministry of Health, the Spanish Respiratory Society (SEPAR), the Catalonian Cardiology Society, Esteve-Teijin (Spain), Oxigen Salud (Spain), and ALLER to develop the ISAACC clinical trial (NCT01335087). None of these funding organizations have participated in the design of the study, data collection, data analysis nor in the interpretation of the data. All other authors declare that they have no conflicts of interest.
Ethical Statement: The ethics committee of each participating center approved the study (approval number in the coordinator center: 2010-852), and subjects provided written informed consent.
References
- Martinez CH, Mannino DM, Jaimes FA, et al. Undiagnosed obstructive lung disease in the United States. Associated factors and long-term mortality. Ann Am Thorac Soc 2015;12:1788-95. [Crossref] [PubMed]
- Mannino DM, Ford ES, Reed SC. Obstructive and restrictive lung disease and markers of inflammation: data from the third national health and nutrition examination. Am J Med 2003;114:758-62. [Crossref] [PubMed]
- Ford ES, Mannino DM, Wheaton AG, et al. Trends in the prevalence of obstructive and restrictive lung function among adults in the United States. Findings from the National Health and Nutrition Examination Surveys from 1988-1994 to 2007-2010. Chest 2013;143:1395-406. [Crossref] [PubMed]
- Garcia-Aymerich J, Serra Pons I, Mannino DM, et al. Lung function impairment, COPD hospitalisations and subsequent mortality. Thorax 2011;66:585-90. [Crossref] [PubMed]
- Lee HM, Le H, Lee BT, Lopez VA, Wong ND. Forced vital capacity paired with Framingham Risk Score for prediction of all-cause mortality. Eur Respir J. 2010;36:1002-6. [Crossref] [PubMed]
- Hole DJ, Watt G, Davey-Smith G, et al. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ 1996;313:711. [Crossref] [PubMed]
- Mannino DM, Buist AS, Petty TL, et al. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax 2003;58:388-93. [Crossref] [PubMed]
- Young RP, Hopkins R, Eaton TE. Forced expiratory volume in one second: not just a lung function test but a marker of premature death from all causes. Eur Respir J 2007;30:616-22. [Crossref] [PubMed]
- McAllister DA, Maclay JD, Mills NL, et al. Arterial stiffness is independently associated with emphysema severity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;176:1208-14. [Crossref] [PubMed]
- Bhatt SP, Dransfield MT. Chronic obstructive pulmonary disease and cardiovascular disease. Transl Res 2013;162:237-51. [Crossref] [PubMed]
- Esquinas C, Sanchez-de-la-Torre M, Aldomá A, et al. Spanish Sleep Network. Rationale and methodology of the impact of continuous positive airway pressure on patients with ACS and nonsleepy OSA: the ISAACC trial. Clin Cardiol 2013;36:495-501. [Crossref] [PubMed]
- Deckers JW. Classification of myocardial infarction and unstable angina: a re-assessment. Int J Cardiol 2013;167:2387-90. [Crossref] [PubMed]
- The Global Strategy for the Diagnosis, Management and Prevention of COPD; Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2018. Available online: (accessed 25/05/2018).http://goldcopd.org
- National Health Interview Survey. Centers for Disease Control and Prevention (CDC) 2017. National Center for Health Statistics. Available from: (accessed 28/05/2018).https://www.cdc.gov/nchs/nhis/tobacco/tobacco_glossary.htm
- García-Río F, Calle M, Burgos F, et al. Espirometría (Spirometry). Arch Bronconeumol 2013;49:388-401. [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. Standardization of spirometry. Eur Respir J 2005;26:319-38. [Crossref] [PubMed]
- Spirometry in Practice – A Practical Guide; British Thoracic Society (BTS) 2005. Available online: (accessed 31/05/2018).https://www.brit-thoracic.org.uk/document-library/delivery-of-respiratory-care/spirometry/spirometry-in-practice-a-practical-guide-(2005)/
- Roca J, Burgos F, Sunyer J, et al. Reference values for forced spirometry. Eur Resp J 1998;11:1354-62. [Crossref]
- Asthma: diagnosis, monitoring and chronic asthma management; National Institute for Health and Care Excellence (NICE) guideline 2017. Available online: (accessed 24/05/2018).https://www.nice.org.uk/guidance/ng80
- Cochet AA, Lucero PF, Zacher LL, Morris MJ. Prevalence of supranormal pulmonary function test values between a military and nonmilitary cohort. Respiratory Care 2014;59:749-55. [Crossref] [PubMed]
- Killip T, Kimball J. Treatment of myocardial infarction in a coronary care unit: a two year experience with 250 patients. Am J Cardiol 1967;20:457-64. [Crossref] [PubMed]
- Long RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr 2006;7:79-108. [Crossref] [PubMed]
- Spanish National Consensus in Sleep Apnea-Hypopnoea Syndrome (SAHS). Arch Bronconeumol 2005;41 Suppl 4:3-11. [PubMed]
- Chiner E, Arriero JM, Signes-Costa J, et al. Validación de la versión española del test de somnolencia Epworth en pacientes con síndrome de apnea del sueño (Validation of the Spanish version of the Epworth Sleepiness Scale in patients with a sleep apnea syndrome). Arch Bronconeumol 1999;35:422-7. [Crossref] [PubMed]
- Speizer FE, Fay ME, Dockery DW, et al. Chronic obstructive pulmonary disease mortality in six U.S. cities. Am Rev Respir Dis 1989;140:S49-55. [Crossref] [PubMed]
- Schunemann HJ, Dorn J, Grant BJ, et al. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest 2000;118:656-64. [Crossref] [PubMed]
- Hospers JJ, Postma DS, Rijcken B, et al. Histamine airway hyper-responsiveness and mortality from chronic obstructive pulmonary disease: a cohort study. Lancet 2000;356:1313-7. [Crossref] [PubMed]
- Min KB, Min JY. Reduced lung function, C-reactive protein, and increased risk of cardiovascular mortality. Circ J 2014;78:2309-16. [Crossref] [PubMed]
- Sin DD, Paul Man SF. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Am Thorac Soc 2005;2:8-11. [Crossref] [PubMed]
- Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003;107:1514-9. [Crossref] [PubMed]
- Gan WQ, Man SF, Senthilselvan A, et al. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax 2004;59:574-80. [Crossref] [PubMed]
- Sabater-Lleal M, Mälarstig A, Folkersen L, et al. Common genetic determinants of lung function, subclinical atherosclerosis and risk or coronary artery disease. PLoS One 2014;9. [Crossref] [PubMed]
- Engström G, Hedblad G, Janzon L. Reduced lung function predicts increased fatality in future cardiac events. A population-based study. J Intern Med 2006;260:560-7. [Crossref] [PubMed]
- Engström G, Wollmer P, Hedlblad B, et al. Occurrence and prognostic significance of ventricular arrhythmias is related to pulmonary function. A study from “Men born in 1914”, Malmö, Sweden. Circulation 2001;103:3086-91. [Crossref] [PubMed]
- Manolio TA, Furberg CD, Rautaharju PM, et al. Cardiac arrhythmias on 24-h ambulatory electrocardiography in older women and men: the Cardiovascular Health Study. J Am Coll Cardiol 1994;23:916-25. [Crossref] [PubMed]
- Berger S, Aronson D, Lavie P, et al. Endothelial progenitor cells in acute myocardial infarction and sleep-disordered breathing. Am J Respir Crit Care Med 2013;187:90-8. [Crossref] [PubMed]
- Angeja BG, Kermgard S, Chen MS, et al. The smoker’s paradox: insights from the angiographic substudies of the TIMI trials. J Thromb Thrombolysis 2002;13:133-9. [Crossref] [PubMed]
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948-68. [Crossref] [PubMed]


