Massive hemoptysis in pulmonary infections: bronchial artery embolization
Massive hemoptysis is a life threatening condition in patients with pulmonary infections, requiring immediate attention via a systematic and multidisciplinary approach utilizing intensivists, pulmonologists, thoracic surgeons, and interventional radiologists. While in certain cases surgery can be the definitive cure, most patients are too acutely ill or have significant underlying medical comorbidities to withstand emergent surgery (1). Bronchial artery embolization (BAE) is a procedure first described in the early 1970’s for the treatment of massive hemoptysis and has since been demonstrated to be safe and effective (2,3). Here we will review the background, diagnostic and clinical considerations, technique, and utility of BAE in patients presenting with massive hemoptysis.
Background: definitions, etiology and pathophysiology
Hemoptysis is defined as expectoration of blood from the lower respiratory tract (4). The definition of massive hemoptysis varies in the literature and often relies on quantitative estimates of expectorated blood which can be challenging to accurately estimate. Expectorated blood volume totaling 300–600 mL over a 24-hour period or three or more bouts of 100 mL blood expectorated over three days in a one week period is considered significant (4-7). In practice, massive hemoptysis is defined as any volume of blood that is life threatening, potentially leading to airway obstruction and asphyxiation as opposed to exsanguination (5,8). Even with modern advances in supportive care and intervention, studies have estimated the mortality of massive hemoptysis to range between 6.5–38% (8-14).
The etiology of massive hemoptysis has varied over time, especially as the incidence of tuberculosis declines in the developed world. The most common causes of massive hemoptysis include bronchiectasis, tuberculosis, mycetomas, necrotizing pneumonia, lung cancer, and cryptogenic hemoptysis (8,9). Worldwide, however, tuberculosis remains the most common cause of massive hemoptysis and can be seen in both cavitary and non-cavitary tuberculosis (1,8) (Figure 1). While malignancy and chronic inflammatory conditions represent a rising share of patients with massive hemoptysis in the Western world, infectious causes including tuberculosis and fungal infections still represent a substantial proportion of these patients (1,8,9). In a 2012 French study which reviewed 1,087 patients presenting with massive hemoptysis, 25% of patients had an underlying history of tuberculosis and 6% presented with aspergillosis. The authors note that patients whose underlying etiology was malignancy or aspergillosis had higher odds of in-hospital mortality (9).
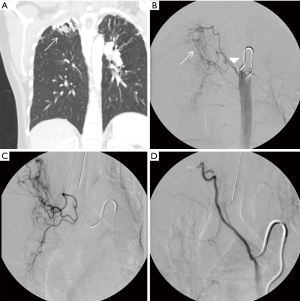
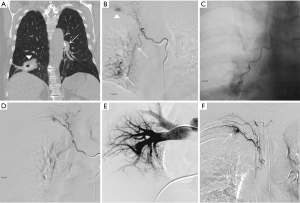
The anatomic source of bleeding in hemoptysis may either be from the pulmonary arteries, which account for approximately 99% of the blood supply to the lungs, or the bronchial arteries which account for approximately 1% (15). Despite the significant difference in volume, 90% of massive hemoptysis originates from the bronchial circulation and only 5% from the pulmonary circulation. The remaining 5% is from the aorta or other systemic arteries (1,8,16). For a variety of reasons, the bronchial arteries are the dominant source in massive hemoptysis: (I) bronchial arteries are subject to higher arterial pressure as opposed to the lower venous pressure of pulmonary arteries; (II) pulmonary circulation is reduced in many pulmonary disease states, which results in vasodilation of the normal bronchial-pulmonary arterial anastomoses resulting in thin walled vessels prone to rupture under arterial pressure; and (III) inflammatory conditions can cause the release of angiogenic factors resulting in neovascularity with vessels that are also prone to rupture (1,8,16,17) (Figure 2).
Diagnostic and clinical considerations
The initial management of patients presenting with hemoptysis centers on stabilization with a primary focus on airway protection. Once a patient has been stabilized, an accurate history and physical exam can be performed to exclude hematemesis and upper airway causes of bleeding. Laboratory testing can be performed to evaluate for inflammatory markers as well as coagulopathy. If a patient is being considered for potential BAE, localization is paramount in order to facilitate safe and effective embolization. Chest radiography is readily available and can be quickly performed; however findings are often normal or not helpful in localization in a large percentage of patients (1,8). Fiberoptic bronchoscopy (FOB) is often the next diagnostic choice of procedure as it is valuable in localization and potentially therapeutic using vasoactive agents, thermal ablative techniques, or bronchial blockers. FOB is also valuable in selectively intubating the uninvolved lung to prevent asphyxiation and has been shown to localize bleeding in 49–92.9% of patients with hemoptysis (1,8,10,18,19). CT has also been validated as a technique for elucidating the cause of hemoptysis as well as localization of the site of bleeding (1,8). Revel et al. reviewed chest radiographs, CT scans, and bronchoscopy findings in 80 patients with massive hemoptysis and found that chest radiographs found the site of bleeding and etiology in 46% and 35% respectively, bronchoscopy found the site and etiology in 73% and 8% respectively, whereas CT found the site and etiology in 70% and 77% respectively (20). As a result, some authors have advocated replacing bronchoscopy with CT, especially in patients who do not require selective intubation (1). At our institution we advocate performing CT angiography (CTA) especially if bronchoscopy cannot be performed because in addition to localization and cause of bleeding, CTA can also map out the location and variability of the bronchial circulation, as well as contribution of other systemic or even pulmonary arteries (21). While we appreciate the benefit of CT, bronchoscopy is an invaluable tool due its potential therapeutic benefit and ability to localize bleeding in patients with diffuse lung disease. In order to mitigate the risk of contrast induced nephropathy, we commonly avoid performing a CTA in patients with compromised renal function, as contrast would be used for both the CT and BAE.
Anatomic considerations
The bronchial arteries run parallel with bronchi and supply the trachea, bronchi, and vasa vasorum of the pulmonary arteries and most often originate directly off the descending thoracic aorta between the levels of T5 and T6 (1,17,22). Bronchial arteries have numerous branching patterns, but four are found to be the most common. In approximately 41% of patients, bronchial arteries present as a single right artery via an intercostobronchial trunk (ICBT) and paired left arteries, which is referred to as a Type I branching pattern. In approximately 21% of patients, a Type II branching occurs when there is a single right bronchial artery via ICBT and a single left bronchial artery. In another 20% of patients a Type III branching is identified, with paired right bronchial arteries (one via ICBT) and paired left bronchial arteries. A type IV branching pattern found in approximately 10% of patients with paired right bronchial arteries (one via ICBT) and a solitary left bronchial artery (1,15,23,24). Bronchial arteries can also originate outside of the T5/6 vertebral level and are considered to be aberrant arteries typically originating from the thyrocervical trunk, brachiocephalic artery, pericardiacophrenic artery, inferior phrenic artery, or abdominal aorta (25,26). These aberrant vessels do not course along the bronchi, but instead run via the pulmonary ligament or adherent pleura with prevalence of 8.3% to 35% (25). Understanding the anatomic bronchial artery variants and aberrant vascular supply is imperative in performing a successful embolization. CTA is helpful in identifying this anatomy prior to performing the procedure.
Technique
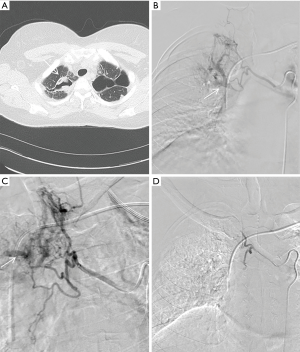
BAE is performed by an interventional radiologist in an angiography suite utilizing both ultrasound and fluoroscopic guidance. The procedure begins by performing a descending thoracic aortogram through a 5 French pigtail catheter via a transfemoral, transbrachial or transradial access, to look for the origin of the bronchial arteries as well as other potential systemic arteries. An aortogram can be avoided in order to save time and decrease iodinated contrast dose if a CTA of the chest has already been performed. Bronchial artery selection is performed using 4 or 5 French catheters (Mikaelson, Cobra, Simmons, Headhunter type catheters) and opacified using iodinated contrast material. Extravasation of iodinated contrast during angiography is an exceedingly rare finding, but more commonly found is hypertrophied bronchial arteries, shunting into the pulmonary vasculature, hypervascularity, or aneurysm formation (1,5,27,28) (Figure 3). Prior to embolization, angiograms must be carefully examined for the presence of medullary arteries which reinforce the anterior spinal artery. Of note, the greater anterior medullary artery or the artery of Adamkiewicz, arises at the T9-T12 level in 75% of cases (1). Anterior medullary arteries classically have a hairpin configuration and can arise from the bronchial or intercostal arteries (1). Once an abnormal bronchial artery is identified, a microcatheter is introduced coaxially through the initial 4 or 5 French catheter and advanced distally to ensure stability and avoid potential anterior medullary branches arising proximally off the bronchial arteries or ICBT. Embolization is most commonly performed using polyvinyl alcohol particles, microspheres, or gelatin sponge suspended in iodinated contrast under direct fluoroscopic guidance in order to prevent reflux or non-target embolization (5,27). Large size particle selection, >300 µm, is preferred in order to prevent necrosis in the bronchial arteries or shunting into the pulmonary circulation via occult anastomoses (27,29). Coil embolization is generally avoided as these occlude the proximal arteries preventing repeat BAE if required. Liquid embolic agents such as cyanoacrylate glue are generally avoided as they can cause distal embolization and require experience to use safely (27,29). The embolization is continued until there is near stasis of particle/contrast elution or complete occlusion of the target vessel.
Outcomes and potential complications
BAE has been shown to be an effective, safe procedure for the treatment of moderate to massive hemoptysis with a technical success rate reported between 81–100% in a recent systematic review (29). BAE has a high immediate clinical success rate reported to range between 70–99%, however the recurrence rate varies widely between 9.8% and 57.5% when evaluating for long term control (29). Early rebleeding following BAE was seen in patients with non-bronchial systemic collaterals, bronchopulmonary shunting, or incomplete embolization (29,30). Late reoccurrences were thought to be due to recanalization of embolized arteries, collaterals supplying the bleeding lesion, or disease progression (29,31). Aspergillomas, reactivation and multi-drug resistent tuberculosis, and malignancy have been reported to have higher bleeding recurrences rates following BAE (29,31-34).
Complications from BAE are overall limited. Transverse myelitis from spinal cord ischemia is the most serious complication typically resulting from non-target embolization of anterior medullary spinal arteries (1,3,5,27). This complication is uncommon, although not rare, and thus patients who would derive the most benefit should be carefully selected to undergo BAE. The reported prevalence of spinal cord ischemia ranges between 1.4% and 6.5% (1,12,27,28,30,31,34,35). Chest pain is the most common complaint following BAE, reported in 1.4–34.5% of patients (5,29). Many of these complications can be prevented through superselective catheterization, larger particle sizes, direct visualization under fluoroscopy, and judicious use of iodinated contrast media. Other complications include vascular injuries such as vasospasm, dissection, arterial puncture site injuries, or dysphagia related to non-target embolization of esophageal feeders (1,29).
Conclusions and future directions
Overall, BAE is a safe and effective procedure for the treatment of moderate to massive hemoptysis of varying etiologies, and can be used as a first line treatment in select patients who are unable to undergo surgical intervention. Experience continues to grow in the use of liquid embolic agents such n-Butyl-2-cyanoacrylate (NBCA) and antireflux microcatheter systems which could potentially increase the safety profile and longer term efficacy. Additionally, the utilization of three dimensional image guidance and intraprocedural cone-beam CT will aid in reducing procedure time and earlier identification of culprit vessels. With proper patient selection and timely intervention, BAE can significantly improve clinical outcomes in patients presenting with hemoptysis of numerous etiologies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yoon W, Kim JK, Kim YH, et al. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics 2002;22:1395-409. [Crossref] [PubMed]
- Remy J, Voisin C, Ribet M, et al. Treatment, by embolization, of severe or repeated hemoptysis associated with systemic hypervascularization. Nouv Presse Med 1973;2:2060. [PubMed]
- Sopko DR, Smith TP. Bronchial artery embolization for hemoptysis. Semin Intervent Radiol 2011;28:48-62. [Crossref] [PubMed]
- Burke CT, Mauro MA. Bronchial artery embolization. Semin Intervent Radiol 2004;21:43-8. [Crossref] [PubMed]
- Kalva SP. Bronchial artery embolization. Tech Vasc Interv Radiol 2009;12:130-8. [Crossref] [PubMed]
- Corey R, Hla KM. Major and massive hemoptysis: reassessment of conservative management. Am J Med Sci 1987;294:301-9. [Crossref] [PubMed]
- Noë GD, Jaffé SM, Molan MP. CT and CT angiography in massive haemoptysis with emphasis on pre-embolization assessment. Clin Radiol 2011;66:869-75. [Crossref] [PubMed]
- Radchenko C, Alraiyes AH, Shojaee S. A systematic approach to the management of massive hemoptysis. J Thorac Dis 2017;9:S1069-86. [Crossref] [PubMed]
- Fartoukh M, Khoshnood B, Parrot A, et al. Early prediction of in-hospital mortality of patients with hemoptysis: an approach to defining severe hemoptysis. Respiration 2012;83:106-14. [Crossref] [PubMed]
- Hirshberg B, Biran I, Glazer M, et al. Hemoptysis: etiology, evaluation, and outcome in a tertiary referral hospital. Chest 1997;112:440-4. [Crossref] [PubMed]
- Knott-Craig CJ, Oostuizen JG, Rossouw G, et al. Management and prognosis of massive hemoptysis. Recent experience with 120 patients. J Thorac Cardiovasc Surg 1993;105:394-7. [PubMed]
- Mal H, Rullon I, Mellot F, et al. Immediate and long-term results of bronchial artery embolization for life-threatening hemoptysis. Chest 1999;115:996-1001. [Crossref] [PubMed]
- Ong TH, Eng P. Massive hemoptysis requiring intensive care. Intensive Care Med 2003;29:317-20. [Crossref] [PubMed]
- Lee TW, Wan S, Choy DK, et al. Management of massive hemoptysis: a single institution experience. Ann Thorac Cardiovasc Surg 2000;6:232-5. [PubMed]
- Walker CM, Rosado-de-Christenson ML, Martínez-Jiménez S, et al. Bronchial arteries: anatomy, function, hypertrophy, and anomalies. Radiographics 2015;35:32-49. [Crossref] [PubMed]
- Remy J, Remy-Jardin M, Voisin C. Endovascular management of bronchial bleeding. In: Butler J. editor. The Bronchial Circulation. New York: Dekker, 1992:667-723.
- Deffebach ME, Charan NB, Lakshminarayan S, et al. The bronchial circulation. Small, but a vital attribute of the lung. Am Rev Respir Dis 1987;135:463-81. [PubMed]
- Hsiao EI, Kirsch CM, Kagawa FT, et al. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. AJR Am J Roentgenol 2001;177:861-7. [Crossref] [PubMed]
- Abal AT, Nair PC, Cherian J. Haemoptysis: aetiology, evaluation and outcome--a prospective study in a third-world country. Respir Med 2001;95:548-52. [Crossref] [PubMed]
- Revel MP, Fournier LS, Hennebicque AS, et al. Can CT replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or massive hemoptysis? AJR Am J Roentgenol 2002;179:1217-24. [Crossref] [PubMed]
- Gupta M, Srivastava DN, Seith A, et al. Clinical impact of multidetector row computed tomography before bronchial artery embolization in patients with hemoptysis: a prospective study. Can Assoc Radiol J 2013;64:61-73. [Crossref] [PubMed]
- Marshall TJ, Jackson JE. Vascular intervention in the thorax: bronchial artery embolization for haemoptysis. Eur Radiol 1997;7:1221-7. [Crossref] [PubMed]
- CAULDWELL EW, SIEKERT RG, et al. The bronchial arteries; an anatomic study of 150 human cadavers. Surg Gynecol Obstet 1948;86:395-412. [PubMed]
- Pump KK. Distribution of bronchial arteries in the human lung. Chest 1972;62:447-51. [Crossref] [PubMed]
- Sancho C, Escalante E, Domínguez J, et al. Embolization of bronchial arteries of anomalous origin. Cardiovasc Intervent Radiol 1998;21:300-4. [Crossref] [PubMed]
- Wong ML, Szkup P, Hopley MJ. Percutaneous embolotherapy for life-threatening hemoptysis. Chest 2002;121:95-102. [Crossref] [PubMed]
- Dave BR, Sharma A, Kalva SP, et al. Nine-year single-center experience with transcatheter arterial embolization for hemoptysis: medium-term outcomes. Vasc Endovascular Surg 2011;45:258-68. [Crossref] [PubMed]
- Wang GR, Ensor JE, Gupta S, et al. Bronchial artery embolization for the management of hemoptysis in oncology patients: utility and prognostic factors. J Vasc Interv Radiol 2009;20:722-9. [Crossref] [PubMed]
- Panda A, Bhalla AS, Goyal A. Bronchial artery embolization in hemoptysis: a systematic review. Diagn Interv Radiol 2017;23:307-17. [Crossref] [PubMed]
- Yu-Tang Goh P, Lin M, Teo N, et al. Embolization for hemoptysis: a six -year review. Cardiovasc Intervent Radiol 2002;25:17-25. [Crossref] [PubMed]
- Fruchter O, Schneer S, Rusanov V, et al. Bronchial artery embolization for massive hemoptysis: long-term follow-up. Asian Cardiovasc Thorac Ann 2015;23:55-60. [Crossref] [PubMed]
- Hwang HG, Lee HS, Choi JS, et al. Risk factors influencing rebleeding after bronchial artery embolization on the management of hemoptysis associated with pulmonary tuberculosis. Tuberc Respir Dis (Seoul) 2013;74:111-9. [Crossref] [PubMed]
- van den Heuvel MM, Els Z, Koegelenberg CF, et al. Risk factors for recurrence of haemoptysis following bronchial artery embolisation for life-threatening haemoptysis. Int J Tuberc Lung Dis 2007;11:909-14. [PubMed]
- Ramakantan R, Bandekar VG, Gandhi MS, et al. Massive hemoptysis due to pulmonary tuberculosis: control with bronchial artery embolization. Radiology 1996;200:691-4. [Crossref] [PubMed]
- Tanaka N, Yamakado K, Murashima S, et al. Superselective bronchial artery embolization for hemoptysis with a coaxial microcatheter system. J Vasc Interv Radiol 1997;8:65-70. [Crossref] [PubMed]


