Esophageal cancer: staging system and guidelines for staging and treatment
Introduction
The incidence of esophageal cancer is increasing, with an estimated 17,460 new cases in the United States in 2012 (1-5). More than 90% of esophageal cancers in the United States are either adenocarcinomas (57%) or squamous cell carcinomas (37%) (1-3,6). The distribution of tumor types varies according to race: 64% of cases in whites are adenocarcinomas, while 82% are of squamous cell origin among the black population (6). Interestingly, the incidence among white males has almost doubled while the incidence among blacks has decreased by almost 50% (6). Tobacco use and a history of mediastinal radiation are risk factors for both tumor types (2). Other risk factors for adenocarcinoma include gastroesophageal reflux disease (GERD), obesity, and Barrett’s esophagus (2). Barrett’s esophagus with high-grade dysplasia is considered a premalignant condition as 50% are found to harbor occult malignant disease at time of biopsy (7). Additional risk factors for squamous cell carcinoma are conditions that cause chronic esophageal irritation and inflammation such as alcohol abuse, achalasia, esophageal diverticuli, and frequent consumption of extremely hot beverages (2). Approximately three quarters of all adenocarcinomas are found in the distal esophagus whereas squamous-cell carcinomas are more evenly distributed throughout the distal two thirds (2).
Obtaining accurate pre-treatment staging and then subsequently providing stage-appropriate treatment is crucial in optimizing esophageal cancer outcomes. Overall 5-year survival for patients with esophageal cancer remains poor, although some improvement has been achieved with an increase from 5% to 17-19% over the past four decades (4-6). These survival improvements have likely resulted from earlier detection in the setting of Barrett’s esophagus, improvements in perioperative care, and the use of adjuvant and induction chemotherapy and radiation. However esophageal cancer treatment and particularly esophagectomy is also associated with significant morbidity. Accurate staging and appropriate treatment can avoid both inadequate and unnecessary treatment to balance the potential benefits of improving prognosis with risks of treatment-related morbidity.
Staging system and guidelines
Staging definitions
Esophageal cancer staging is defined by the American Joint Committee on Cancer (AJCC) Staging System that establishes tumor-node-metastasis (TNM) sub-classifications based on the depth of invasion of the primary tumor (T), lymph node involvement (N), and extent of metastatic disease (M). The most recent, 7th edition of the AJCC Cancer Staging Manual for esophagus and esophagogastric junction cancers was developed based on a database of 4,627 esophagectomy patients who were not treated with induction or adjuvant therapy (8). This data from 13 institutions in five countries and three continents was collected by the Worldwide Esophageal Cancer Collaboration (WECC) (9). Table 1 shows the specific 7th edition TNM definitions. The 7th edition differed from the 6th edition in several respects (10,11). The T status classification was changed to define Tis as high-grade dysplasia and all non-invasive neoplastic epithelium. Tumors with T4 status due to invasion of local structures were subdivided into tumors that involved resectable local structures such as pleura and diaphragm (T4a) and unresectable local structures such as aorta and vertebral bodies (T4b).
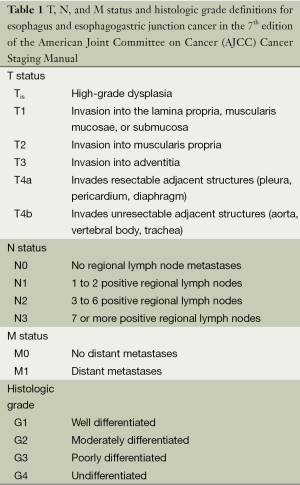
Full table
Regional lymph nodes were also redefined as any paraesophageal lymph node, including cervical or celiac nodes. The N status had been categorized simply as node-negative or node-positive in the 6th edition and was redefined in the 7th edition to N0-N3 based on the number of lymph nodes. The M1a and M1b subclassifications from the 6th edition were redefined to M1. The 7th edition stage groupings were also defined to consider the importance of histopathologic cell type, tumor grade, and tumor location. Table 2 shows stage grouping for adenocarcinoma and squamous cell carcinoma, which are no longer equivalent in the 7th edition.
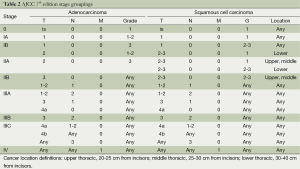
Full table
Diagnostic and staging work-up
The Society of Thoracic Surgeons has published guidelines on the diagnosis and staging of patients with esophageal cancer (12). The work-up for esophageal cancer often starts when patients present with symptoms such as dysphagia and weight loss in the setting of an unremarkable physical exam (2,13). Therefore, the most common tests used to initially identify and diagnosis esophageal cancer are upper gastrointestinal (GI) tract contrast studies and upper endoscopy with biopsy. An upper GI contrast study typically shows a stricture or ulceration when malignancy is present. Upper GI endoscopy identifies tumor location and length and allows biopsy for pathologic examination. After a histologic cancer diagnosis has been obtained, subsequent studies are performed to determine clinical stage as accurately as possible before treatment is initiated.
Obtaining a computed tomographic (CT) scan of the chest and abdomen with both oral and intravenous contrast should be the first staging study when esophageal cancer is diagnosed histologically. The CT scan is somewhat limited in defining the local extent and nodal involvement of esophageal cancer but is most useful in identifying the presence of distant disease such as liver or lung metastases. Further studies that evaluate T and N status would not typically impact treatment and therefore are generally unnecessary if distant disease is identified and subsequently confirmed by biopsy. Positron-emission tomography (PET) scans improve staging by detecting previously unsuspected metastatic disease in up to 15-20% of patients and should be considered in place of CT scans or as an additional study when the CT scan does not show metastatic disease (14,15).
If CT and PET do not demonstrate distant disease, endoscopic ultrasound (EUS) should be performed to establish the extent of locoregional disease (2). EUS provides more accurate evaluation of the depth of tumor invasion (T status) and the extent of lymph-node involvement (N status) than both PET and CT (16,17). However, EUS is less accurate for early-stage lesions such as T1 or T2 compared to more advanced tumors (18-21). Most incidences of understaging are due to missing nodal disease. The specificity and the sensitivity for identifying lymph node disease are better when EUS is combined with fine-needle aspiration (FNA) compared to EUS alone (22).
Performance of the above staging modalities establishes the pre-treatment clinical stage which can be used to guide subsequent treatment, as will be discussed in the following sections. However, occasionally additional studies may be worthwhile before initiation of treatment. First, bronchoscopy should be considered for tumors in the upper and middle esophagus to rule out airway invasion. CT scan and EUS can be suggestive of airway involvement but are not as accurate as direct visualization of the airway. In addition, distant metastases are unfortunately missed even with completion of the staging evaluation described above. Small liver or lung metastases can be missed by both PET and CT scans, and patients can also have undetected pleural or peritoneal disease (23). Staging via minimally invasive surgical techniques of thoracoscopy and laparoscopy improves the accuracy of the above non-invasive testing (23-25). Use of these invasive techniques is relatively uncommon but should be considered in select patients, such as those who may be considered to have a high risk of treatment-related complications. Staging laparoscopy in particular may have a role for patients with adenocarcinoma of the esophagus or esophagogastric junction (26).
Treatment guidelines
The National Comprehensive Cancer Network (NCCN) provides guidelines for the treatment of esophageal cancer (27). Treatment options include local mucosal resection or ablation therapies, esophagectomy, chemotherapy, and radiation therapy. Recommended treatment is primarily dictated by stage, tumor location, and patients’ medical fitness for receiving a particular therapeutic modality. However, definitive data from randomized trials to guide the treatment of esophageal cancer is lacking for many clinical situations. Outcomes also generally are relatively poor with many treatment strategies, so establishing optimal treatment for different clinical situations remains an area of active research (28). The NCCN guidelines reflect the lack of definitive evidence and often allow a spectrum of potential treatments for many clinical situations. Given both the generally poor overall prognosis and the potential morbidity associated with therapy, multidisciplinary evaluation by surgery, medical oncology, and radiation oncology should be considered for all patients before a treatment strategy is initiated. Treatment that does not follow guidelines should probably only be used in the context of clinical trials.
The stage groupings described above are very useful for both providing prognosis and guiding treatment. However, patients can be categorized even more simply when considering treatment. When considering treatment for esophageal cancer patients, the approach is initially dictated by whether the patients have been determined to have early stage superficial cancers, cancers that are locally advanced with locoregional disease but no distant metastases, and cancers with distant disease. The general treatment guidelines for each of these categories will be discussed in the following sections.
Superficial cancers
Patients with T1-2N0 esophageal cancer typically are recommended to undergo surgery without induction treatment (27). The prognosis for patients treated for intra- and submucosal (T1) esophageal cancers is significantly better than the prognosis for all other patients found to have esophageal cancer, even those also found in other relatively early-stage disease (8). Esophagectomy is effective oncologically for these cancers, but is associated with considerable morbidity and mortality despite improvements over time and the development of minimally invasive techniques (29-35). Although recent data from high-volume centers have shown low mortality rates of 1% to 3.5%, studies involving population-based databases or multi-center trials show that esophageal resection continues to have relatively high perioperative mortality rates of 8.8% to 14% (30,32,35-37). Local treatments with modalities such as endoscopic mucosal resection, radiofrequency ablation, cryotherapy, and photodynamic therapy can provide effective cancer treatment for superficial cancers with much less treatment-related morbidity (38-50). These local treatments are good treatment options for patients with superficial tumors that involve only the mucosa (T1a), but close endoscopic surveillance should be planned post-treatment. However, local mucosal therapies at the present time are generally not considered appropriate for superficial tumors that involve the submucosa (T1b), as these lesions have occulted lymph node involvement in as many as 50% of patients (51,52). Therefore esophagectomy without induction therapy is recommended for superficial tumors that involve the submucosa (T1b),
The optimal management of esophageal cancer clinically staged as T2N0M0 is somewhat more controversial (53). Clinical staging modalities for this subset are somewhat unreliable, with significant percentages of patients being both under and over staged (18,54-57). Perhaps because clinical staging inaccuracies lead to a relatively high incidence of patients actually having nodal disease present at the time of surgical resection, induction therapy use in this setting has been increasing and was shown recently to exceed 50% for cases that were reported to the Society of Thoracic Surgeons General Thoracic Database in 2011 (54). However, data that proves a survival benefit to induction therapy over surgery alone is still lacking (58). Consistent with the uncertainty of optimal treatment, the NCCN guidelines for medically fit patients allow a wide spectrum of treatment possibilities that include definitive chemoradiation and esophagectomy with or without induction or adjuvant therapy (27).
Locoregional or locally advanced disease
Approximately 32% of esophageal cancer patients have regional disease at the time of diagnosis, with a 5-year survival of only 10-30% (1,2,8). The treatment for locally advanced esophageal cancer that does not have distant metastases and is potentially resectable (T3-4aN0, T1-4aN1M0) is highly variable in practice (59). The NCCN guidelines reflect a lack of available definitive data on the optimal treatment and essentially consider any combination of esophagectomy and chemoradiation or even definitive chemoradiation as acceptable therapy (27).
Many studies involving various combinations of surgery, chemotherapy, and radiation to treat locally advanced esophageal cancer have been conducted and showed conflicting results (28,37,60-66). However, recent evidence suggests that induction chemoradiation followed by surgical resection is the optimal treatment for patients with T3-4a tumors or nodal disease. Several recent trials, retrospective studies, and meta-analyses all showed a survival benefit to both combined and induction therapy (67-72). Most importantly, a recently published randomized trial demonstrated a survival benefit to induction chemoradiation followed by surgery compared to surgery alone for esophageal or esophagogastric junction cancer (73).
Radiation alone followed by surgery does not improve survival compared with surgery alone and therefore induction radiation alone is not recommended (27,65). Induction chemotherapy without radiation has variably shown to be beneficial but is used by some high-volume centers, and is recommended as a potential treatment by the NCCN for patients with adenocarcinoma (27,37,64). Definitive chemoradiation is the preferred treatment for patients with T4b (unresectable) tumors and occasionally can facilitate surgical resection in selected cases.
Metastatic or unresectable disease
Approximately 50% of patients have evidence of distant metastatic disease at the time of diagnosis (2,6). Palliative therapy is recommended for these patients, and can include chemotherapy, clinical trial enrollment if available, or best supportive care. Best supportive care is often the most appropriate treatment option. Patients’ performance status should determine whether chemotherapy is added to best supportive care. Specific symptoms that often need palliation include dysphagia, pain, and nausea. Oncologists often are hesitant to pursue feeding tubes in patients with stage IV cancer, but feeding tubes may be reasonable options in some select patients. Radiation or endoscopic management techniques such as dilation and stenting can be used to palliate dysphagia or cases of bleeding from esophageal tumors. Palliative esophagectomy for patients with metastatic disease may have a role in very few cases, but should be considered only in very select cases given the morbidity of surgery and the poor prognosis with or without surgery.
Other considerations
Role of esophagectomy for esophageal cancer
Concurrent chemoradiation is an effective treatment option for patients with squamous cell carcinoma of the cervical esophageal cancer (74-77). The NCCN guidelines recommend definitive chemoradiation for these patients (27). Surgery is recommended as possible treatment for most other cases of esophageal cancer that do not have invasion of unresectable structures or distant metastatic disease. Esophageal resection can be performed via several different techniques, with the most appropriate technique for any specific individual patient being dependent on both patient and surgeon factors. Several studies have suggested that complete surgical resection provides the best chance for cure in patients who do not have distant disease (64,78,79). For patients with stage I-III disease who receive surgical treatment, 5-year survival is 28%, compared to 10% for those treated medically (78). However, surgery for locoregional esophageal cancer is utilized in only 30-40% of resectable cases, perhaps because esophagectomy is historically associated with significant morbidity and mortality and disappointing long-term results (78,80). Minimizing perioperative morbidity in any manner possible is critical to increase the use of surgical resection so that primary nonsurgical treatment is reserved for those who refuse surgery, have unresectable cancers, or are not thought to be surgical candidates for other reasons.
Squamous cell carcinoma versus adenocarcinoma
Squamous cell carcinoma was previously the most common histology but now accounts for 37% of esophageal cancers (1,3). Adenocarcinoma is now the most common esophageal cancer. Patients with adenocarcinoma and squamous cell carcinoma have been observed to have similar long-term survival across major treatment modalities, suggesting that both histologies respond similarly to treatment and may share significant physiologic and cellular features (81). Accordingly, staging and treatment guidelines for adenocarcinoma and squamous cell carcinoma were previously essentially equivalent. However, recognition of prognosis and response to treatment between the two subtypes led to separate stage groupings and treatment algorithms in the latest, revised staging system and in the NCCN guidelines (8,27).
Esophageal cancer treatment guidelines are still generally similar to both adenocarcinoma and squamous cell carcinoma (27). However, the benefit of surgical resection in improving survival compared to definitive chemoradiation for esophageal squamous cell carcinoma has been questioned (82). In particular, several randomized trials have suggested that definitive chemoradiation could offer equivalent survival to treatment that involves surgery for locally advanced, non-metastatic esophageal SCC (83-85). Currently for medically fit patients with resectable disease, the NCCN treatment guidelines only recommend definitive chemoradiation for patients who decline surgery (27). However, some centers advocate treatment with chemoradiation for esophageal squamous cell carcinoma, with surgery subsequently used only when there is persistent or recurrent local disease (86).
Adjuvant therapy
Adjuvant therapy after resection may have a role for some esophageal cancer patients. Postoperative radiation may reduce the incidence of local recurrence in those patients who have residual tumor after resection but is not beneficial in the absence of residual disease (2,87,88). Postoperative chemotherapy has not been definitively shown to have an additive effect on survival compared with surgery alone although additional therapy may be warranted in patients who have a high likelihood of metastatic disease based on a large number of tumor positive nodes (89). The NCCN does not recommend adjuvant therapy if patients have a had a complete R0 resection for squamous cell carcinoma, but does recommend consideration of adjuvant chemoradiation, or only adjuvant chemotherapy if induction radiation was administered, for patients who have had resection of adenocarcinoma with either node-positive disease or T2-T4a tumors (27). The guidelines also recommend consideration of adjuvant therapy in the setting of microscopic or macroscopic residual disease after resection.
Conclusions
Survival of esophageal cancer is improving but remains poor. Esophageal cancer stage is based on depth of tumor invasion, involvement of regional lymph nodes, and the presence of metastatic disease. Most patients present with either locally advanced or metastatic disease. Appropriate work-up is critical to identify accurate pre-treatment staging so that both under-treatment and unnecessary treatment is avoided. Staging evaluation should start with CT or PET scan, and patients who do not have metastatic disease should have EUS to determine the locoregional extent of disease. Treatment strategy should follow guideline recommendations, and generally should be developed after multidisciplinary evaluation. Surgery or local mucosal treatments should be considered for superficial cancers. Multimodality therapy that includes surgery is generally considered the best treatment for locally advanced cancers, while patients that have metastatic disease should be considered for chemotherapy along with best supportive care.
Acknowledgements
Dr. Berry has received support from the National Institute of Health (NIH) funded Cardiothoracic Surgical Trials Network. There are no disclosures or potential conflicts to report.
Disclosure: The author declares no conflict of interest.
References
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2008, National Cancer Institute. Bethesda, MD. Available online: http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011. 2011.
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [PubMed]
- Pennathur A, Luketich JD. Resection for esophageal cancer: strategies for optimal management. Ann Thorac Surg 2008;85:S751-6. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [PubMed]
- Dubecz A, Gall I, Solymosi N, et al. Temporal trends in long-term survival and cure rates in esophageal cancer: a SEER database analysis. J Thorac Oncol 2012;7:443-7. [PubMed]
- Horner M, Ries L, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2006, National Cancer Institute. Bethesda, MD. Available online: http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site, 2009.
- Nigro JJ, Hagen JA, DeMeester TR, et al. Occult esophageal adenocarcinoma: extent of disease and implications for effective therapy. Ann Surg 1999;230:433-8. [PubMed]
- Rice TW, Rusch VW, Ishwaran H, et al. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer 2010;116:3763-73.
- Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus 2009;22:1-8. [PubMed]
- Rice TW, Blackstone EH, Rusch VW. 7th Edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric Junction. Ann Surg Oncol 2010;17:1721-4.
- Greene FL, Page DL, Fleming ID, et al. eds. AJCC Cancer Staging Manual. 6th ed. New York: Springer-Verlag, 2003.
- Varghese TK Jr, Hofstetter WL, Rizk NP, et al. The society of thoracic surgeons guidelines on the diagnosis and staging of patients with esophageal cancer. Ann Thorac Surg 2013;96:346-56. [PubMed]
- Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg 2000;190:562-72. [PubMed]
- Downey RJ, Akhurst T, Ilson D, et al. Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol 2003;21:428-32. [PubMed]
- Luketich JD, Schauer PR, Meltzer CC, et al. Role of positron emission tomography in staging esophageal cancer. Ann Thorac Surg 1997;64:765-9. [PubMed]
- Choi J, Kim SG, Kim JS, et al. Comparison of endoscopic ultrasonography (EUS), positron emission tomography (PET), and computed tomography (CT) in the preoperative locoregional staging of resectable esophageal cancer. Surg Endosc 2010;24:1380-6. [PubMed]
- Sandha GS, Severin D, Postema E, et al. Is positron emission tomography useful in locoregional staging of esophageal cancer? Results of a multidisciplinary initiative comparing CT, positron emission tomography, and EUS. Gastrointest Endosc 2008;67:402-9. [PubMed]
- Rice TW, Mason DP, Murthy SC, et al. T2N0M0 esophageal cancer. J Thorac Cardiovasc Surg 2007;133:317-24. [PubMed]
- Kutup A, Link BC, Schurr PG, et al. Quality control of endoscopic ultrasound in preoperative staging of esophageal cancer. Endoscopy 2007;39:715-9. [PubMed]
- Pech O, Günter E, Dusemund F, et al. Accuracy of endoscopic ultrasound in preoperative staging of esophageal cancer: results from a referral center for early esophageal cancer. Endoscopy 2010;42:456-61. [PubMed]
- DeWitt J, Kesler K, Brooks JA, et al. Endoscopic ultrasound for esophageal and gastroesophageal junction cancer: impact of increased use of primary neoadjuvant therapy on preoperative locoregional staging accuracy. Dis Esophagus 2005;18:21-7. [PubMed]
- Vazquez-Sequeiros E, Wiersema MJ, Clain JE, et al. Impact of lymph node staging on esophageal carcinoma therapy. Gastroenterology 2003;125:1626-35. [PubMed]
- Luketich JD, Friedman DM, Weigel TL, et al. Evaluation of distant metastases in esophageal cancer: 100 consecutive positron emission tomography scans. Ann Thorac Surg 1999;68:1133-6. [PubMed]
- Krasna MJ, Reed CE, Jaklitsch MT, et al. Thoracoscopic staging of esophageal cancer: a prospective, multiinstitutional trial. Cancer and Leukemia Group B Thoracic Surgeons. Ann Thorac Surg 1995;60:1337-40. [PubMed]
- Krasna MJ, Flowers JL, Attar S, et al. Combined thoracoscopic/laparoscopic staging of esophageal cancer. J Thorac Cardiovasc Surg 1996;111:800-6. [PubMed]
- de Graaf GW, Ayantunde AA, Parsons SL, et al. The role of staging laparoscopy in oesophagogastric cancers. Eur J Surg Oncol 2007;33:988-92. [PubMed]
- Ajani JA, Barthel JS, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw 2011;9:830-87. [PubMed]
- D’Amico TA. Surgery for esophageal cancer. Gastrointest Cancer Res 2008;2:S6-9. [PubMed]
- Chang LC, Oelschlager BK, Quiroga E, et al. Long-term outcome of esophagectomy for high-grade dysplasia or cancer found during surveillance for Barrett’s esophagus. J Gastrointest Surg 2006;10:341-6. [PubMed]
- Bailey SH, Bull DA, Harpole DH, et al. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg 2003;75:217-22; discussion 222. [PubMed]
- Chang AC, Ji H, Birkmeyer NJ, et al. Outcomes after transhiatal and transthoracic esophagectomy for cancer. Ann Thorac Surg 2008;85:424-9. [PubMed]
- Rentz J, Bull D, Harpole D, et al. Transthoracic versus transhiatal esophagectomy: a prospective study of 945 patients. J Thorac Cardiovasc Surg 2003;125:1114-20. [PubMed]
- Connors RC, Reuben BC, Neumayer LA, et al. Comparing outcomes after transthoracic and transhiatal esophagectomy: a 5-year prospective cohort of 17,395 patients. J Am Coll Surg 2007;205:735-40. [PubMed]
- Dimick JB, Wainess RM, Upchurch GR, et al. National trends in outcomes for esophageal resection. Ann Thorac Surg 2005;79:212-6. [PubMed]
- Ra J, Paulson EC, Kucharczuk J, et al. Postoperative mortality after esophagectomy for cancer: development of a preoperative risk prediction model. Ann Surg Oncol 2008;15:1577-84. [PubMed]
- Berry MF, Atkins BZ, Tong BC, et al. A comprehensive evaluation for aspiration after esophagectomy reduces the incidence of postoperative pneumonia. J Thorac Cardiovasc Surg 2010;140:1266-71. [PubMed]
- Orringer MB, Marshall B, Chang AC, et al. Two thousand transhiatal esophagectomies: changing trends, lessons learned. Ann Surg 2007;246:363-72. [PubMed]
- Soetikno R, Kaltenbach T, Yeh R, et al. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol 2005;23:4490-8. [PubMed]
- Galey KM, Wilshire CL, Watson TJ, et al. Endoscopic management of early esophageal neoplasia: an emerging standard. J Gastrointest Surg 2011;15:1728-35. [PubMed]
- Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut 2008;57:1200-6. [PubMed]
- Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137:815-23. [PubMed]
- Chennat J, Konda VJ, Ross AS, et al. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma--an American single-center experience. Am J Gastroenterol 2009;104:2684-92. [PubMed]
- Ciocirlan M, Lapalus MG, Hervieu V, et al. Endoscopic mucosal resection for squamous premalignant and early malignant lesions of the esophagus. Endoscopy 2007;39:24-9. [PubMed]
- Larghi A, Lightdale CJ, Ross AS, et al. Long-term follow-up of complete Barrett’s eradication endoscopic mucosal resection (CBE-EMR) for the treatment of high grade dysplasia and intramucosal carcinoma. Endoscopy 2007;39:1086-91. [PubMed]
- Sibille A, Lambert R, Souquet JC, et al. Long-term survival after photodynamic therapy for esophageal cancer. Gastroenterology 1995;108:337-44. [PubMed]
- Corti L, Skarlatos J, Boso C, et al. Outcome of patients receiving photodynamic therapy for early esophageal cancer. Int J Radiat Oncol Biol Phys 2000;47:419-24. [PubMed]
- Tanaka T, Matono S, Nagano T, et al. Photodynamic therapy for large superficial squamous cell carcinoma of the esophagus. Gastrointest Endosc 2011;73:1-6. [PubMed]
- Fujita H, Sueyoshi S, Yamana H, et al. Optimum treatment strategy for superficial esophageal cancer: endoscopic mucosal resection versus radical esophagectomy. World J Surg 2001;25:424-31. [PubMed]
- Greenstein AJ, Wisnivesky JP, Litle VR. Effect of local therapy for the treatment of superficial esophageal cancer in non-operative candidates. Dis Esophagus 2008;21:673-8. [PubMed]
- Berry MF, Zeyer-Brunner J, Castleberry AW, et al. Treatment modalities for T1N0 esophageal cancers: a comparative analysis of local therapy versus surgical resection. J Thorac Oncol 2013;8:796-802. [PubMed]
- Nigro JJ, Hagen JA, DeMeester TR, et al. Prevalence and location of nodal metastases in distal esophageal adenocarcinoma confined to the wall: implications for therapy. J Thorac Cardiovasc Surg 1999;117:16-23. [PubMed]
- Rice TW, Zuccaro G Jr, Adelstein DJ, et al. Esophageal carcinoma: depth of tumor invasion is predictive of regional lymph node status. Ann Thorac Surg 1998;65:787-92. [PubMed]
- Kountourakis P, Correa AM, Hofstetter WL, et al. Combined modality therapy of cT2N0M0 esophageal cancer: the University of Texas M. D. Anderson Cancer Center experience. Cancer 2011;117:925-30. [PubMed]
- Crabtree TD, Kosinski AS, Puri V, et al. Evaluation of the reliability of clinical staging of T2 N0 esophageal cancer: a review of the Society of Thoracic Surgeons database. Ann Thorac Surg 2013;96:382-90. [PubMed]
- Crabtree TD, Yacoub WN, Puri V, et al. Endoscopic ultrasound for early stage esophageal adenocarcinoma: implications for staging and survival. Ann Thorac Surg 2011;91:1509-15. [PubMed]
- Stiles BM, Mirza F, Coppolino A, et al. Clinical T2-T3N0M0 esophageal cancer: the risk of node positive disease. Ann Thorac Surg 2011;92:491-6. [PubMed]
- Zhang JQ, Hooker CM, Brock MV, et al. Neoadjuvant chemoradiation therapy is beneficial for clinical stage T2 N0 esophageal cancer patients due to inaccurate preoperative staging. Ann Thorac Surg 2012;93:429-35. [PubMed]
- Martin JT, Worni M, Zwischenberger JB, et al. The role of radiation therapy in resected T2 N0 esophageal cancer: a population-based analysis. Ann Thorac Surg 2013;95:453-8. [PubMed]
- Smith GL, Smith BD, Buchholz TA, et al. Patterns of care and locoregional treatment outcomes in older esophageal cancer patients: the SEER-Medicare Cohort. Int J Radiat Oncol Biol Phys 2009;74:482-9. [PubMed]
- Graham AJ, Shrive FM, Ghali WA, et al. Defining the optimal treatment of locally advanced esophageal cancer: a systematic review and decision analysis. Ann Thorac Surg 2007;83:1257-64. [PubMed]
- Fiorica F, Di Bona D, Schepis F, et al. Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta-analysis. Gut 2004;53:925-30. [PubMed]
- Kaklamanos IG, Walker GR, Ferry K, et al. Neoadjuvant treatment for resectable cancer of the esophagus and the gastroesophageal junction: a meta-analysis of randomized clinical trials. Ann Surg Oncol 2003;10:754-61. [PubMed]
- Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer 2005;103:1347-55. [PubMed]
- Kelsen DP, Winter KA, Gunderson LL, et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol 2007;25:3719-25. [PubMed]
- Arnott SJ, Duncan W, Gignoux M, et al. Preoperative radiotherapy for esophageal carcinoma. Cochrane Database Syst Rev 2005;CD001799. [PubMed]
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7. [PubMed]
- Worni M, Castleberry AW, Gloor B, et al. Trends and outcomes in the use of surgery and radiation for the treatment of locally advanced esophageal cancer: a propensity score adjusted analysis of the surveillance, epidemiology, and end results registry from 1998 to 2008. Dis Esophagus 2013. [Epub ahead of print]. [PubMed]
- Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 2005;6:659-68. [PubMed]
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [PubMed]
- Schwer AL, Ballonoff A, McCammon R, et al. Survival effect of neoadjuvant radiotherapy before esophagectomy for patients with esophageal cancer: a surveillance, epidemiology, and end-results study. Int J Radiat Oncol Biol Phys 2009;73:449-55. [PubMed]
- Solomon N, Zhuge Y, Cheung M, et al. The roles of neoadjuvant radiotherapy and lymphadenectomy in the treatment of esophageal adenocarcinoma. Ann Surg Oncol 2010;17:791-803. [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [PubMed]
- Burmeister BH, Dickie G, Smithers BM, et al. Thirty-four patients with carcinoma of the cervical esophagus treated with chemoradiation therapy. Arch Otolaryngol Head Neck Surg 2000;126:205-8. [PubMed]
- Wang S, Liao Z, Chen Y, et al. Esophageal cancer located at the neck and upper thorax treated with concurrent chemoradiation: a single-institution experience. J Thorac Oncol 2006;1:252-9. [PubMed]
- Uno T, Isobe K, Kawakami H, et al. Concurrent chemoradiation for patients with squamous cell carcinoma of the cervical esophagus. Dis Esophagus 2007;20:12-8. [PubMed]
- Tong DK, Law S, Kwong DL, et al. Current management of cervical esophageal cancer. World J Surg 2011;35:600-7. [PubMed]
- Paulson EC, Ra J, Armstrong K, et al. Underuse of esophagectomy as treatment for resectable esophageal cancer. Arch Surg 2008;143:1198-203. [PubMed]
- Abrams JA, Buono DL, Strauss J, et al. Esophagectomy compared with chemoradiation for early stage esophageal cancer in the elderly. Cancer 2009;115:4924-33. [PubMed]
- Dubecz A, Sepesi B, Salvador R, et al. Surgical resection for locoregional esophageal cancer is underutilized in the United States. J Am Coll Surg 2010;211:754-61. [PubMed]
- Chang DT, Chapman C, Shen J, et al. Treatment of esophageal cancer based on histology: a surveillance epidemiology and end results analysis. Am J Clin Oncol 2009;32:405-10. [PubMed]
- Yamashita H, Nakagawa K, Yamada K, et al. A single institutional non-randomized retrospective comparison between definitive chemoradiotherapy and radical surgery in 82 Japanese patients with resectable esophageal squamous cell carcinoma. Dis Esophagus 2008;21:430-6. [PubMed]
- Bedenne L, Michel P, Bouche O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160-8. [PubMed]
- Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005;23:2310-7. [PubMed]
- Chiu PW, Chan AC, Leung SF, et al. Multicenter prospective randomized trial comparing standard esophagectomy with chemoradiotherapy for treatment of squamous esophageal cancer: early results from the Chinese University Research Group for Esophageal Cancer (CURE). J Gastrointest Surg 2005;9:794-802. [PubMed]
- Castoro C, Scarpa M, Cagol M, et al. Complete clinical response after neoadjuvant chemoradiotherapy for squamous cell cancer of the thoracic oesophagus: is surgery always necessary? J Gastrointest Surg 2013;17:1375-81. [PubMed]
- Fok M, Sham JS, Choy D, et al. Postoperative radiotherapy for carcinoma of the esophagus: a prospective, randomized controlled study. Surgery 1993;113:138-47. [PubMed]
- Ténière P, Hay JM, Fingerhut A, et al. Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle and lower esophagus as shown by a multicenter controlled trial. French University Association for Surgical Research. Surg Gynecol Obstet 1991;173:123-30. [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. Predicting systemic disease in patients with esophageal cancer after esophagectomy: a multinational study on the significance of the number of involved lymph nodes. Ann Surg 2008;248:979-85. [PubMed]


