Age modification of ozone associations with cardiovascular disease risk in adults: a potential role for soluble P-selectin and blood pressure
Introduction
There has been considerable variation in the observed associations between ozone (O3) and cardiovascular outcomes across studies (1). It is possible that these variations are in part due to differences in the physical characteristics (e.g., age) of the study populations. Some epidemiological studies have suggested that older age could increase the risk of O3-associated mortality based on categorization of individuals above or below an “elderly” age cutoff of between 54–85 years old (2-4). However, other studies have failed to observe the effect modification of O3 associations with mortality by age (5,6). In terms of preclinical outcomes, the modification of O3 effects by age in humans has not been previously explored for cardiovascular effects, although one study found that older age increased one’s susceptibility to O3-induced lung function decrements (7).
Recently we reported that an increase in both 24-hour and 2-week O3 exposure was associated with increases in the platelet activation marker plasma soluble sCD62P (8) and blood pressure in a cohort of 89 healthy participants who lived in a work campus in Changsha City, China (9). We refer to this study as the Changsha Study in the current paper. Similarly, in another study, which we refer to as the Shanghai Study, we also measured O3 exposure (as well as co-pollutants), sCD62P, and blood pressure in a cohort of 71 healthy participants who worked and resided in a medical campus in Shanghai, China. The age ranges were 22 to 52 years old in the Changsha Study and 19–26 years old in the Shanghai Study. Using the data collected in these two studies, we aim in this paper to examine potential age modifications in the association of O3 with sCD62P and blood pressure. Based on the findings from the present analysis and the relevant literature, we also postulate a mechanistic model for the age-dependent cardiovascular effects of O3 exposure.
Methods
The Changsha study
The methods for this study have been described previously (9). Briefly, 89 healthy white-collar workers living and working on a work campus in suburban Changsha, China, were recruited after being screened for pre-existing self-reported health conditions and blood markers of liver, kidney, or metabolic dysfunction. Participants underwent four measurements each separated by about 2 weeks for plasma P-selectin (sCD62P) and brachial systolic and diastolic blood pressure (SBP and DBP). Additional biomarkers and physiologic outcomes were also measured but are not reported here as they are not relevant to the current analysis (9). Indoor O3 and particulate matter less than 2.5 µm in diameter (PM2.5) monitoring for offices and living spaces was combined with outdoor O3, PM2.5, NO2, and SO2 monitoring and 24-hour time-activity data to calculate individual exposures to O3 and co-pollutants. Given that there were at least 2 weeks between each biomarker sampling period, 2-week exposures were also estimated as a measure of “sub-chronic” exposures (versus 24-hour exposures) using previously described methods (9).
The Shanghai study
The methods of this study have been previously reported (10). Briefly, this study was conducted with 71 medical students recruited from the suburban southern campus of the Shanghai First People’s Hospital. All the participants attended classes and lived in the same dormitory building on the campus. Grounds for exclusion included self-reported major chronic diseases, smoking, living in the same room as a smoker, and abnormal blood levels of markers of kidney, liver, and metabolic function. Participants were randomly assigned to undergo biomarker sampling over two weekends, each separated by 2 weeks, between Nov. 7th and Dec. 14th, 2015.
Indoor O3 concentrations were estimated based on ambient O3 data from a monitoring station 23 km away indoor/outdoor (I/O) ratios were estimated depending on dormitory room filtration conditions and ventilation as estimated using CO2 measurements. An estimated I/O ratio of 0.35 was used for all other indoor environments. PM2.5 exposure assessment for this study was calculated using continuous PM2.5 concentrations measured in each dormitory room, one representative classroom and clinical office, and outdoors with laser photometers (Beijing Green Built Environment Technology Co., Ltd., Model QD11, Beijing, China) throughout the study period. The 24-hour time-activity questionnaires similar to those used in the Changsha Study were used to estimate 24-hour PM2.5 and O3 exposures. Health outcomes including plasma sCD62P and blood pressure were measured for each participant on two weekends separated at least by 2 weeks. During each of these two weekends, fasting plasma samples were collected between 7:00 and 8:00 AM on Saturday, Sunday, and the following Monday, whereas SBP and DBP were measured between 7:30 AM and 3:30 PM on Saturday and Sunday.
SBP and DBP were measured using the same instrument (VICORDER®, SMT Medical, Würzberg, Germany) in both the Changsha and Shanghai studies. However, plasma sCD62P was analyzed by our research staff using commercial ELISA kits in the Changsha Study (9) and by a commercial laboratory (Merck Chemicals Co., Ltd., Shanghai, China) using the Merck Milliplex® MAP assay (Merck Millipore, USA) in the Shanghai study.
Statistical analysis
In order to reduce the possibility of variance inflation due to potentially high collinearity between predictor main effects, interaction terms, and covariates, we utilized a Bayesian hierarchical generalized ridge regression (GRR) mixed effects model. Participant-specific intercepts were used as random effects in the model to account for the correlation in the within-person repeated measures. This model utilized the “shrinkage priors” of a penalized Cauchy distribution for mean coefficient estimates and a half-Cauchy distribution for the standard deviation of the random intercepts. These prior distributions have previously been shown to improve maximum likelihood estimation when there is high collinearity between predictor variables (11), which was verified with simulations using our own data. The variables included in the model are shown in Eq. [1], where 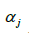 is the random intercept for subject j, the first three predictors are the main effects for O3 and age and the interaction term for observation i and subject j,
is the random intercept for subject j, the first three predictors are the main effects for O3 and age and the interaction term for observation i and subject j, 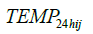 and
and 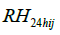 are the 24-hour mean ambient temperature and relative humidity,
are the 24-hour mean ambient temperature and relative humidity,  indicates the time spent in a room with an active smoker in the past 24 hours as a measure of secondhand smoke, RIS is respiratory infection status, MS is menstruation status, WD is day of the week, LA and is the hours since a subject last ate.
indicates the time spent in a room with an active smoker in the past 24 hours as a measure of secondhand smoke, RIS is respiratory infection status, MS is menstruation status, WD is day of the week, LA and is the hours since a subject last ate.
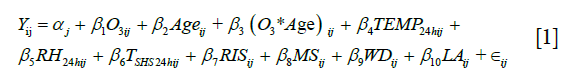
These models were used to test for interactions between age and either 24-hour or 2-week mean O3 exposure using the Changsha Study data. Only 24-hour mean exposure data were available for the Shanghai Study data, which were also analyzed using the GRR model. The data for these two studies were then pooled together to assess the robustness of age interactions with 24-hour O3 exposure across multiple studies. The same Bayesian GRR model used on each individual study was utilized for the pooled data, with the only difference being the addition of a binary fixed effect variable for study to control for possible differences in mean biomarker values between the two studies. The distribution of sCD62P data was right-skewed, and hence sCD62P concentrations were log-transformed.
To check that any significant interaction terms were independent of the main effects, we regressed the interaction term on its two main effects in a frequentist linear mixed model and then used the residuals from that model as a predictor in the Bayesian GRR models. If the original analysis shows a significant interaction term but this secondary analysis does not, it is likely that the main effects are highly correlated with and are confounding the interaction term, and so any apparent association with the interaction term may just be due to one or both of the main effects. Additional sensitivity analyses included omitting data from active smokers, omitting extreme outliers, and controlling for all other pollutant exposures. The available co-pollutant data included PM2.5 for the Changsha Study, the Shanghai Study, and the pooled analyses. In addition, NO2 and SO2 concentration data were available for the Changsha Study. Furthermore, the influence of gender on either the O3 main effect or O3 by age interaction term was evaluated. Additional sensitivity analysis methodological details are in the Supplementary material.
The predicted marginal effect of the exposure predictor over the subject age range was calculated for each model with a significant interaction. This was done by using the posterior samples of the coefficient estimates from the Bayesian models to calculate the conditional coefficient for the exposure predictor (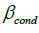 ) using the formula
) using the formula 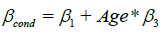 , based on the definitions of
, based on the definitions of 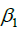 and
and 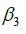 in Eq. [1]. All reported 95% credible intervals (95% CIs) are highest posterior density (HPD) intervals. All calculations were made using JAGS (12), version 4.2.0, and the “R2jags” (13) and “R2WinBUGS” (14) packages in R, version 3.3.3 (15).
in Eq. [1]. All reported 95% credible intervals (95% CIs) are highest posterior density (HPD) intervals. All calculations were made using JAGS (12), version 4.2.0, and the “R2jags” (13) and “R2WinBUGS” (14) packages in R, version 3.3.3 (15).
Results
Subject characteristics, exposures, and biomarker levels between studies
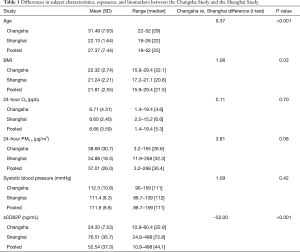
A comparison of variables between the Changsha Study and the Shanghai Study is shown in Table 1. The male to female gender ratio of the Changsha Study, 2.56, is significantly higher (P<0.001) than that of the Shanghai Study, 0.78, and the pooled gender ratio is 1.42. The Changsha Study had a significantly higher mean age by about 9 years. In terms of other potentially confounding participant characteristics, the mean body mass index (BMI) in the Changsha Study was also significantly higher than that of the Shanghai Study, though they both have similar ranges and the mean difference was only about 1 kg/m2. Both studies had comparable 24-hour O3 and PM2.5 exposure concentrations, as well as similar mean SBP values. In addition, all participants from both studies had a similar socioeconomic status (data not shown). However, the Shanghai Study had significantly higher sCD62P values, possibly due to the differences in the measurement methods of ELISA versus multiplex analysis. Another difference between the studies was that the Shanghai Study had no smokers, and there was no secondhand smoke exposure, whereas the Changsha Study had 15 (17%) active smokers and 6 (7%) former smokers.
Full table
Interaction estimates from the Changsha study
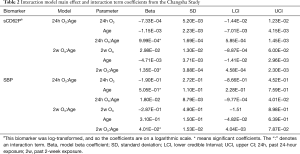
The posterior estimates for main effect and interaction term coefficients in models with significant interactions are highlighted in Table 2. In order to visualize how these interactions influence the association between pollutant exposure and biomarkers, each significant interaction is shown in a marginal effect plot with age on the x-axis and the predicted unit or percent change in each biomarker associated with a 10-ppb increase in O3 exposure on the y-axis. These plots show how the conditional coefficients of the association between exposures and biomarkers change as age changes.
Full table
In terms of age interactions, these analyses showed significant interaction terms of 2-week O3 by age for SBP and 24-hour O3 by age and 2-week O3 by age for sCD62P. Though not reaching statistical significance at a significance level of 0.05, the O3 interaction estimate for 24-hour O3 by age for SBP had a lower credible interval just barely overlapping zero. The O3 main effect estimates all had credible intervals overlapping zero, indicating that at a theoretical age of zero there would be no effect of O3 on the biomarkers, which is consistent with a decreasing effect of O3 as age decreases.
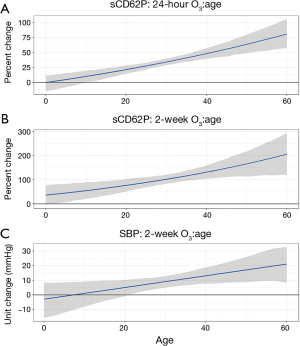
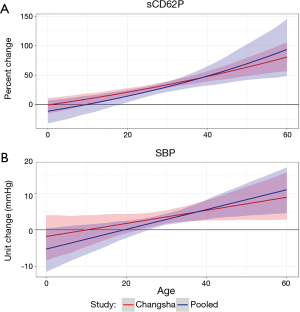
In Figure 1, there is a clear increasing trend in the association between O3 and sCD62P or SBP. At the age of 25, the approximate first quartile of the experimental age range, a 10 ppb increase in 24-hour or 2-week O3 exposure is predicted to increase sCD62P by a mean (95% CI) of 27.5% (21.4%, 32.8%) and 89.0% (68.8%, 111.4%), respectively, and these estimates increase to 48.0% (39.7%, 57.2%) and 131.6% (104.2%, 160.1%), respectively, at age 40, which is the approximate third quartile of the experimental age range. For the association between a 10 ppb increase in 2-week O3 exposure and SBP, a 25-year-old is predicted to have a SBP increase of 6.3 (2.3, 10.0) mmHg, and a 40-year-old is predicted to have an increase of 11.7 (6.9, 16.8) mmHg.
When testing these associations using the residuals of the interaction term regressed on the main effects (see Table S1 in the Supplementary), all of these associations remained significant. In the sensitivity analyses controlling for all pollutant main effects, the 24-hour and 2-week O3 by age interactions for sCD62P remained significant, but the 2-week O3 by age interaction for SBP became nonsignificant. Excluding active smokers from the analysis did not change results, and all O3 by gender and O3 by age by gender interaction terms in the gender sensitivity analysis were nonsignificant and centered at zero.
Full table
Interaction estimates from the Shanghai study
There were no significant interaction term estimates for any of the biomarkers in the analysis of the Shanghai Study data. The main effect and interaction term results are presented in Table S2 in the Supplementary.

Full table
Interaction estimates from the pooled data
After pooling the data, estimates generally had tighter credible intervals and lower effect sizes (see Table 3). In the pooled data analysis, the 24-hour O3 by age interaction estimate for SBP, 0.027 (95% CI: 0.006, 0.047), increased and became significant. The sCD62P association with the 24-hour O3 by age interaction term increased and remained significant [0.0013 (95% CI: 0.0006, 0.0021)].

Full table
Figure 2 juxtaposes the conditional 24-hour O3 exposure coefficients and credible intervals for the Changsha Study analysis results (shown in red) and the pooled data analysis results (shown in blue). Figure 2A,B show that the slope of the change in conditional pollutant coefficient is greater in the pooled analysis, and this difference in slopes is more noticeable for SBP. For the pooled analysis, a 10 ppb increase in 24-hour O3 exposure is predicted to be associated with a 22.3% (14.3%, 31.2%) and 48.6% (32.7%, 65.1%) increase in sCD62P for a 25- and 40-year-old, respectively. For SBP, the increases predicted to be associated with a 10 ppb increase in 24-hour O3 exposure are 1.35 (−0.18, 2.84) mmHg and 4.98 (2.56, 7.35) mmHg for a 25- and 40-year-old, respectively. These interaction terms did not change significance in any of the sensitivity analyses, and none of the O3 by gender or O3 by age and gender interaction terms were significant (Tables S1,S3 in the Supplementary).
Full table
Discussion
The present analysis suggests an increased vulnerability to O3-associated increases in biomarkers of cardiovascular disease pathophysiology in older individuals. In particular, there were significant, robust interactions between age and O3 in association with sCD62P and SBP that were detectable both when evaluating the Changsha Study alone and when combining the data from that study and another study with a younger cohort. We summarize the evidence for the role of platelets, blood pressure, and aging in O3 cardiovascular effects below.
Aging and ozone modifications to platelet activation and blood pressure
Many of the mechanisms involved in the regulation of platelet activation are known to be modified by age. Multiple studies have found decreases in the thresholds required for ADP-induced platelet aggregation as well as decreased fibrinolytic activity in platelets harvested from older individuals (16). This increased reactivity with age may be in part due to platelets in older people having a higher expression of mRNA for genes related to platelet activation, α-granule secretion, and other thrombotic processes, including genes encoding VWF and the α-granule-associated thrombotic factor platelet factor 4 (PF4) (17). In addition, the plasma of older individuals contains higher basal levels of circulating hemostatic factors, including VWF, fibrinogen, and plasminogen activator inhibitor-1 (PAI-1) (18), as well as factors directly associated with the release of platelet α-granules where sCD62P is stored, such as PF4 (19) and β-thromboglobulin (20). It is unclear if older individuals have increased basal sCD62P, with a significant age-associated increase observed in older rats (21) but not in humans (22). Furthermore, older mice have increased platelet hydrogen peroxide, a platelet agonist, in addition to increased platelet NADPH oxidase, which produces hydrogen peroxide (23).
The age-related increases in factors that lead to platelet activation and aggregation also may influence blood pressure by increasing sensitivity to vasoconstriction. Prostaglandin I2 (PGI2) acts both as a vasodilator and also an inhibitor of platelet activation, but in older individuals there is lower platelet surface density of PGI2 receptors (24) and increased urinary PGI2 metabolite concentrations, indicating increased production to compensate for decreased sensitivity (25). In addition, urinary metabolite concentrations of the platelet-derived vasoconstrictor and platelet activator TXA2 were also found to be increased in older individuals (25). Endothelial nitric oxide synthase (eNOS) and the nitric oxide it produces inhibit platelet activation and induces vasodilation, and eNOS activity has been shown to be reduced in older healthy rats (26). Also, platelets take up and store circulating serotonin and release it when they aggregate, and it has been shown that older women have higher levels of platelet serotonin (27). Both platelet activation and blood pressure increases are primed to occur in older individuals as a result of these changes in molecular signaling.
Evidence of ozone effects on platelet and blood pressure-associated mechanisms affected by age
Many of the mechanisms behind age-associated increases in platelet activation and blood pressure have also previously been associated with O3 exposure. Of the aforementioned circulating hemostatic factors that are known to be increased with age, ambient O3 levels have been associated with fibrinogen (28-30), VWF (29), and PAI-1 (28) in humans, though these associations have also been nonsignificant in other human studies evaluating the same biomarkers (31-33). In addition, O3 exposure in mice has been shown to increase mRNA expression for genes encoding fibrinogen, VWF, and PAI-1 (34).
In terms of the aforementioned age-modified vasoconstrictive mechanisms, O3 has been associated with decreased eNOS in mice (35) and increased TXB2, the stable metabolite of TXA2, in guinea pigs (36). Furthermore, O3 exposure in rats leads to increased sensitivity to serotonin-induced vasoconstriction that was shown to be both transferrable by blood transfusion and blunted by increasing superoxide dismutase and catalase as well as reducing NADPH oxidase (37). This indicates a key role for superoxide and hydrogen peroxide derived from NADPH oxidase, known to increase with age, in O3-associated vasoconstriction. These studies have suggested that there is considerable overlap between mechanisms involved in age-associated and O3-associated susceptibility to platelet activation and vasoconstriction, which supports our novel observation of an interaction between O3 exposure and age on these cardiovascular pathophysiologic mechanisms.
Limitations
Though the Changsha Study and pooled datasets encompassed a relatively wide age range (22–52 years old), most of the subjects were in their twenties or early thirties, and so there may have been a lack of statistical power in evaluating additional effect modifications by age that might have been detectable with more subjects in their 40s or older. In addition, the age range (19–26 years old) of the Shanghai Study was small, limiting the effectiveness of the data from that study in testing age interactions in the pooled analysis. This was apparent in the Pearson correlation coefficients of 0.98–0.99 between the pollutant exposure variables and interaction terms in the Shanghai Study data, indicating a miniscule effect of age on the interaction term. Furthermore, the differences in the O3 exposure measurement methods between the two studies may have biased results in the pooled analysis. The Changsha Study had actual indoor O3 measurements in addition to exposures estimated with outdoor data and I/O ratios, while the Shanghai Study only had the latter. However, the Pearson correlation coefficient between measured and modeled indoor O3 measurements in the Changsha Study was 0.88, supporting the use of modeled measurements. Building characteristics were taken into account when determining I/O ratios for the Shanghai Study, so it is likely that the modeled O3 measurements in that study were similarly accurate and that the lack of measured indoor O3 data has a minimal risk of inducing substantial bias. Finally, the mechanisms being explored are based on only a few biomarkers. Additional biomarkers would help verify the mechanisms that seem to underlie our results.
Conclusions
This analysis provides the first evidence that age increases adults’ susceptibility to O3-associated increases in platelet activation and blood pressure. Based on previous biochemical and toxicological literature, there is ample evidence to support the hypothesis that mechanisms controlling platelet activation and blood pressure are both more active and more vulnerable to insult in older age. Surprisingly, these age modifications were evident in subjects who were largely still young adults and none of whom were elderly. It is unclear if this effect modification continues increasing into old age. If so, the elderly could be more sensitive to these cardiopulmonary pathophysiologic mechanisms than those who are younger, so this is an area that warrants further study. When estimating the health burden attributable to air pollution or setting standards for acceptable exposure levels, the increased vulnerability of older individuals to cardiovascular effects should be taken into account to prevent underestimation of the health impact of air pollutant exposure.
Supplementary
This supporting information shows additional results for the Shanghai Study analysis and the results of the sensitivity analyses. These sensitivity analyses included using the residuals of a model regressing the interaction term on the main effects as a predictor in the main models, controlling for all co-pollutants, omitting data from active smokers, omitting extreme outliers in the pooled analysis, and evaluating the effect modification of gender on O3 and O3 by age associations with biomarkers. The results are shown in the following sections.
Shanghai study analysis results
There were no significant interaction terms for the analysis of the Shanghai Study data. In the Changsha Study and pooled data analyses, significant ozone by age interaction terms were found in relation to sCD62P and SBP. Below the coefficient estimate results for these two biomarkers from the Shanghai Study analysis are presented in Table S2.
Sensitivity analyses
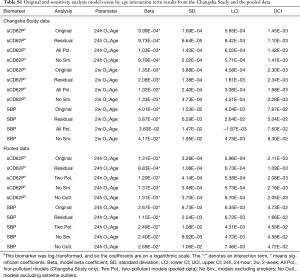
Interaction term model estimates from the main models were checked against a sensitivity analysis using the residuals of a linear mixed model in which the interaction term was regressed on the main ozone and age effects as the predictor in the main Bayesian GRR models. For a given two main effects X1 and X2 and interaction term X3, the formula for obtaining the interaction term residuals would be the linear mixed model X3ij ~ β1X1ij + β2X2ij + αj + εij, where the αj is the j subject-specific intercept random effect and the residuals for subject j and observation i are εij. Table S1 compares the original interaction term estimates to the estimates obtained using this method. In addition, sensitivity analyses controlling for co-pollutants [PM2.5, NO2, and SO2 for the Changsha Study data (“All Pol.”) and PM2.5 for the pooled data (“Two Pol.”)], omitting data from active smokers (“No Sm.”), and omitting extreme outliers in the pooled data (“No Outl.”) were performed using the original statistical approach. Lower and upper extreme outliers were defined as being less than or greater than 3 times the interquartile range (IQR) from the first or third quartile, respectively. The results of those analyses are also presented in Table S1.
Sensitivity analyses assessing the possible influence of gender on the observed ozone associations with biomarkers were also performed. First, the influence of gender on ozone by age interaction effects was assessed by including ozone by age by gender interaction term along with all lower-level main effects and two-way interaction terms in the model. All of the posterior estimates for ozone by age by gender interaction terms had credible intervals overlapping and centered at zero, indicating that gender did not influence the ozone by age interactions. The estimates for the three-way ozone by age by gender interaction terms can be found in Table S3. Furthermore, another analysis replaced the ozone by age interaction term and related main effect terms with ozone by gender interaction term and its related main effect terms to examine if biomarker associations with ozone are influenced by gender when not controlling for the influence of age. The posterior estimates for the two-way ozone by gender interaction terms, all of which had credible intervals overlapping zero, can be seen in Table S3.
Acknowledgements
We thank the Broad Group for providing access to their workers and facilities and the Shanghai General Hospital for providing access to their students and facilities. Neither the Broad Group nor the Shanghai General Hospital had any involvement in the study design or the interpretation of the results.
Funding: This research was supported by a grant from the National Natural Science Foundation of China (51420105010). Dr. Day’s research was also supported by a training grant from the NIEHS (T32-ES021432) and from the Doctoral Scholars Program of the Duke Global Health Institute. Dr. Cui received funding support from the Duke University Integrated Toxicology and Environmental Health Program and Duke University Graduate School.
Footnote
Conflicts of Interest: The authors declare no conflicts of interest. Drs. Y Zhang and J Zhang had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
References
- Brown J, Bowman C. Integrated Science Assessment for Ozone and Related Photochemical Oxidants. Federal Register 2013;78:11172-3.
- Medina-Ramón M, Schwartz J. Who is more vulnerable to die from ozone air pollution? Epidemiology 2008;19:672-9. [Crossref] [PubMed]
- Ruidavets JB, Cournot M, Cassadou S, et al. Ozone air pollution is associated with acute myocardial infarction. Circulation 2005;111:563-9. [Crossref] [PubMed]
- Stafoggia M, Forastiere F, Faustini A, et al. Susceptibility factors to ozone-related mortality: A population-based case-crossover analysis. Am J Respir Crit Care Med 2010;182:376-84. [Crossref] [PubMed]
- Peng RD, Samoli E, Pham L, et al. Acute effects of ambient ozone on mortality in Europe and North America: Results from the APHENA Study. Air Qual Atmos Health 2013;6:445-53. [Crossref] [PubMed]
- Stieb DM, Judek S, Burnett RT. Meta-analysis of time-series studies of air pollution and mortality: Effects of gases and particles and the influence of cause of death, age, and season. J Air Waste Manag Assoc 2002;52:470-84. [Crossref] [PubMed]
- Eckel SP, Louis TA, Chaves PH, et al. Modification of the association between ambient air pollution and lung function by frailty status among older adults in the Cardiovascular Health Study. Am J Epidemiol 2012;176:214-23. [Crossref] [PubMed]
- Blann AD, Nadar SK, Lip GY. The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J 2003;24:2166-79. [Crossref] [PubMed]
- Day DB, Xiang J, Mo J, et al. Association of ozone exposure with cardiorespiratory pathophysiologic mechanisms in healthy adults. JAMA Intern Med 2017;177:1344-53. [Crossref] [PubMed]
- Cui X, Li F, Xiang J, et al. Cardiopulmonary effects of overnight indoor air filtration in healthy non-smoking adults: A double-blind randomized crossover study. Environ Int 2018;114:27-36. [Crossref] [PubMed]
- James G, Witten D, Hastie T. An Introduction to Statistical Learning: With Applications in R. New York: Springer Texts in Statistics; 2014.
- Plummer M. editor. JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling. Proceedings of the 3rd international workshop on distributed statistical computing; 2003: Vienna.
- Su YS, Yajima M. R2jags: Using R to Run 'JAGS'. R package version 0.5-7; 2015.
- Sturtz S, Ligges U, Gelman A. R2WinBUGS: A package for running WinBUGS from R. J Stat Softw 2005;12:1-16. [Crossref]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017.
- Gleerup G, Winther K. The effect of ageing on platelet function and fibrinolytic activity. Angiology 1995;46:715-8. [Crossref] [PubMed]
- Simon LM, Edelstein LC, Nagalla S, et al. Human platelet microRNA-mRNA networks associated with age and gender revealed by integrated plateletomics. Blood 2014;123:e37-45. [Crossref] [PubMed]
- Mohebali D, Kaplan D, Carlisle M, et al. Alterations in platelet function during aging: Clinical correlations with thromboinflammatory disease in older adults. J Am Geriatr Soc 2014;62:529-35. [Crossref] [PubMed]
- Zahavi J, Jones NA, Leyton J, et al. Enhanced in vivo platelet "release reaction" in old healthy individuals. Thromb Res 1980;17:329-36. [Crossref] [PubMed]
- Bastyr EJ 3rd, Kadrofske MM, Vinik AI. Platelet activity and phosphoinositide turnover increase with advancing age. Am J Med 1990;88:601-6. [Crossref] [PubMed]
- Zou Y, Jung KJ, Kim JW, et al. Alteration of soluble adhesion molecules during aging and their modulation by calorie restriction. FASEB J 2004;18:320-2. [Crossref] [PubMed]
- Ponthieux A, Herbeth B, Droesch S, et al. Age- and sex-related reference values for serum adhesion molecule concentrations in healthy individuals: Intercellular adhesion molecule-1 and E-, P-, and L-selectin. Clin Chem 2003;49:1544-6. [Crossref] [PubMed]
- Dayal S, Wilson KM, Motto DG, et al. Hydrogen peroxide promotes aging-related platelet hyperactivation and thrombosis. Circulation 2013;127:1308-16. [Crossref] [PubMed]
- Modesti PA, Fortini A, Abbate R, et al. Age related changes of platelet prostacyclin receptors in humans. Eur J Clin Invest 1985;15:204-8. [Crossref] [PubMed]
- Reilly IA, FitzGerald GA. Eicosenoid biosynthesis and platelet function with advancing age. Thromb Res 1986;41:545-54. [Crossref] [PubMed]
- Chou TC, Yen MH, Li CY, et al. Alterations of nitric oxide synthase expression with aging and hypertension in rats. Hypertension 1998;31:643-8. [Crossref] [PubMed]
- Kumar AM, Weiss S, Fernandez JB, et al. Peripheral serotonin levels in women: Role of aging and ethnicity. Gerontology 1998;44:211-6. [Crossref] [PubMed]
- Chuang KJ, Chan CC, Su TC, et al. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med 2007;176:370-6. [Crossref] [PubMed]
- Liao D, Heiss G, Chinchilli VM, et al. Association of criteria pollutants with plasma hemostatic/inflammatory markers: A population-based study. J Expo Anal Environ Epidemiol 2005;15:319-28. [Crossref] [PubMed]
- Steinvil A, Kordova-Biezuner L, Shapira I, et al. Short-term exposure to air pollution and inflammation-sensitive biomarkers. Environ Res 2008;106:51-61. [Crossref] [PubMed]
- Kahle JJ, Neas LM, Devlin RB, et al. Interaction effects of temperature and ozone on lung function and markers of systemic inflammation, coagulation, and fibrinolysis: A crossover study of healthy young volunteers. Environ Health Perspect 2015;123:310-6. [PubMed]
- Thompson AM, Zanobetti A, Silverman F, et al. Baseline repeated measures from controlled human exposure studies: Associations between ambient air pollution exposure and the systemic inflammatory biomarkers IL-6 and fibrinogen. Environ Health Perspect 2010;118:120-4. [PubMed]
- Zhang J, Zhu T, Kipen H, et al. Cardiorespiratory biomarker responses in healthy young adults to drastic air quality changes surrounding the 2008 Beijing Olympics. Res Rep Health Eff Inst 2013.5-174. [PubMed]
- Kodavanti UP, Thomas R, Ledbetter AD, et al. Vascular and cardiac impairments in rats inhaling ozone and diesel exhaust particles. Environ Health Perspect 2011;119:312-8. [Crossref] [PubMed]
- Chuang GC, Yang Z, Westbrook DG, et al. Pulmonary ozone exposure induces vascular dysfunction, mitochondrial damage, and atherogenesis. Am J Physiol Lung Cell Mol Physiol 2009;297:L209-16. [Crossref] [PubMed]
- Miller PD, Ainsworth D, Lam HF, et al. Effect of ozone exposure on lung functions and plasma prostaglandin and thromboxane concentrations in guinea pigs. Toxicol Appl Pharmacol 1987;88:132-40. [Crossref] [PubMed]
- Paffett ML, Zychowski KE, Sheppard L, et al. Ozone inhalation impairs coronary artery dilation via intracellular oxidative stress: Evidence for serum-borne factors as drivers of systemic toxicity. Toxicol Sci 2015;146:244-53. [Crossref] [PubMed]


