Bronchopleural phrenic fistula caused by methicillin-resistant staphylococcus aureus pneumonia
Introduction
Methicillin-resistant staphylococcus aureus (MRSA) is a common pathogen in hospital-acquired infections, and community-acquired MRSA infection is also increasingly common (1,2). We experienced a patient with community acquired pneumonia due to MRSA and she showed aggravating clinical course in intensive care unit (ICU). There was subphrenic abscess below pneumonic infiltration in Rt. middle and lower lobe. We suspected diaphragmatic defect and bronchoscopy revealed communication between bronchus and subphrenic abscess. Occasionally pulmonologists meet bronchopleural fistula (BPF) cases after pulmonary infections for example tuberculosis, bacterial pneumonia and fungal infection. But we have never seen the bronchopleural phrenic fistula in pneumonia patient before. There are some case reports of invasiveness and fistula formation associated with MRSA infection (3-6), but there was no bronchopleural phrenic fistula until now. Here we report an interesting pneumonia case caused by MRSA with bronchopleural phrenic fistula and was treated successfully without surgical intervention.
Case presentation
A 55-year-old woman visited our emergency department in November 2017. Seven days prior to presentation, she had developed cough, yellowish sputum, and fever, which had progressed to dyspnea at home. She had diabetes mellitus, chronic kidney dysfunction, and neurogenic bladder with a double J stent in her ureters. She had previously suffered recurrent urinary tract infections. Vital signs were assessed, showing the following: blood pressure, 84/47 mmHg; heart rate, 95 bpm; respiratory rate, 30 breaths/min; body temperature, 36.4 °C; and SpO2, 81%. Oxygen (3 L/min) was administered via a nasal prong, and the patient’s systolic blood pressure subsequently recovered to >90 mmHg. Initial laboratory testing of blood samples included the following: arterial blood gas analysis (pH, 7.393; pCO2, 39.0 mmHg; pO2, 72.5 mmHg; HCO3, 23.3 mmol/L; SaO2, 93.6%); lactic acid (1 mmol/L); white blood cell count (38,710/µL); hemoglobin (7.0 g/dL), hematocrit (22.2%); platelet count (370 K/µL); sodium (111 mEq/L); potassium (3.5 mEq/L); chloride (79 mEq/L); total CO2 (21 mEq/L); blood urea nitrogen/creatinine ratio (48.8/3.23 mg/dL); Aspartate transaminase/Alanine transaminase (AST/ALT) (18/11 IU/L); total bilirubin (0.16 mg/dL); protein/albumin (5.7/2.5 g/dL); glucose (432 mg/dL); CRP (18.19 mg/dL; normal levels <0.3 mg/dL); procalcitonin (2.58 ng/mL; normal levels <0.05 ng/mL); and pro-brain natriuretic peptide (BNP) (5,618 pg/mL). Urinalysis results were as follows: protein, 1+; glucose, 2+; blood, 2+; leukocyte, 3+; nitrite, negative. Influenza antigen test was negative, and respiratory virus polymerase chain reaction (PCR) tests were all negative (16 types).
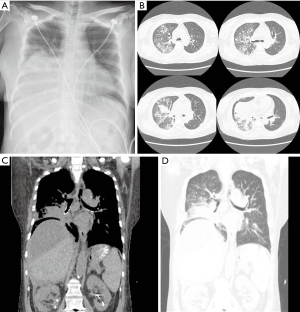
Initial chest X-ray showed diffuse haziness in the right lower lung field (Figure 1). Non-enhanced chest computed tomography (CT) was performed due to concerns regarding deteriorating kidney function. CT scan showed diffuse consolidations in the right middle and lower lobes, multiple nodules in the right upper lobe, and multiple areas of ground glass opacity in the left lung, in addition to fluid above the liver (Figure 1). These results indicated pneumonia with parapneumonic effusion and perihepatic ascites. Intravenous levofloxacin 750 mg was administrated after blood culture, and the patient was admitted to the general ward (GW) 5 hours after presenting in the ER. At this stage, the patient had a fever (39.3 °C), and oxygen was increased to 5 L/min. Respiratory arrest occurred 7 hours after admission; the patient’s mental status was confused, and her pulse was not palpable. Ventricular tachycardia was suspected, and direct current (DC) cardioversion was performed. The patient was intubated and transferred to the ICU.
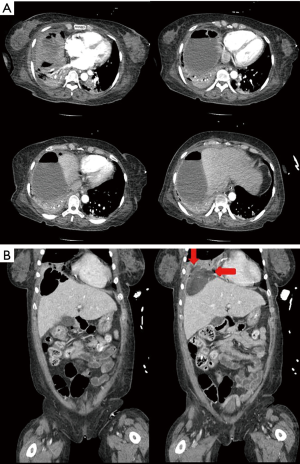
In the ICU, the patient required mechanical ventilation, and piperacillin-tazobactam 2.25 gram was administered in addition to levofloxacin. Her vital signs were subsequently stabilized. Enhanced abdominal and pelvic CT scan showed subphrenic abscess and gas above the liver, but there was no liver abscess or biliary problems (Figure 2). After 8 hours, the patient’s mental status recovered fully. Due to the suspicion of a subphrenic abscess, percutaneous catheter drainage (PCD) was inserted. From which 540 cc of pus was drained, and a Jackson-Pratt (JP) drainage chamber was repeatedly inflated. This condition was thought to be due to air-forming bacteria. Chest X-ray showed aggravated pneumonia, and the fraction of inspired oxygen (FiO2) administered via the mechanical ventilator was increased to 70%.
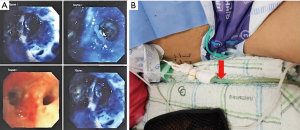
On hospital day (HD) 4, sputum and subphrenic abscess culture revealed MRSA, and vancomycin 1.0 gram was added to the treatment regimen. CRP levels were 30.15 mg/dL. The JP drainage system was changed to a chest bottle, and multiple air bubbles were observed from the PCD. CT scan showed possible defects in the right diaphragm. Bronchoscopy was undertaken to investigate the possibility of a BPF and, therefore, connection to the intra-abdominal cavity via the diaphragm (Figure 3). Diluted methylene blue and saline were introduced into the right middle lobar bronchus; blue-colored fluid was subsequently seen from the PCD in the subphrenic space. We therefore confirmed the presence of bronchopleural phrenic fistula due to MRSA pneumonia. Although a thoracic surgeon was consulted with a view to repairing the diaphragmatic fistula, the patient’s clinical condition was so critical that surgery was considered impossible at that time. Best medical treatment, including antibiotics and supportive care, was therefore undertaken.
On HD 6, pneumonia was seen to have progressed to the left lung. Treatment was switched from piperacillin-tazobactam to meropenem, but the clinical course did not improve. Culture of bronchoscopic aspirates and pus from PCD revealed carbapenem-resistant Acinetobacter baumannii, and as vancomycin did not impact on the MRSA pneumonia, antibiotic therapy was switched to linezolid and colistin on HD 12. From HD 13, the patient’s clinical course began to gradually improve, percutaneous dilatational tracheostomy (PDT) was performed on HD 17. After HD 18, air leakage from the PCD ceased, and we attempted to wean the patient from the mechanical ventilator to pressure support.
On HD 23, CRP levels had improved to 5.68 mg/dL.
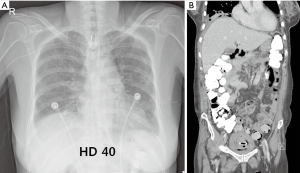
On HD 33, the patient was transferred to the GW, at which point CRP levels were 1.27 mg/dL. Her condition was stable with no recurrence of pneumonia or bronchopleural phrenic fistula (Figure 4). She received physical therapy for ambulation in the GW and was discharged on HD 96.
Discussion
In 1977, cases of bronchobiliary fistula and BPF were first reported during postoperative stricture of the bile duct (7). Many cases of hepatobronchial fistula caused by liver abscess, carcinoma, and parasites have been reported (8-20). By contrast, the case reported here was not associated with hepatobiliary disease.
MRSA is a common pathogen in hospital-acquired infections, and community-acquired MRSA infection is also increasingly common (1,2). Pulsed-field gel electrophoresis can be used to identify the strain of MRSA (21), although typing was not performed in the present case. The patient had suffered three episodes of urinary tract infection in 2017; in two of these episodes, the pathogen was MRSA. There are some case reports of invasiveness and fistula formation associated with MRSA infection: endocarditis caused by MRSA and ruptured myocardial abscess causing left ventricle to pulmonary artery communication in an infant (3), and endocarditis resulting in right ventricular pseudoaneurysm and fistulation to a previous bypass graft in an elderly man (4). Jeannon et al. reported pharyngo-cutaneous fistulas (PCFs) following total laryngectomy (5). A case of BPF secondary to MRSA necrotizing pneumonia was reported in 2017, but this involved BPF only, rather than phrenic fistula (6).
Conclusions
We report a rare case of bronchopleural phrenic fistula caused by MRSA pneumonia. After treatment with PCD and antibiotics, the pneumonia resolved and the diaphragmatic fistula healed spontaneously without the need for surgical intervention.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med 2006;355:666-74. [Crossref] [PubMed]
- Hersh AL, Chambers HF, Maselli JH, et al. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 2008;168:1585-91. [Crossref] [PubMed]
- Hershenson JA, Baker PB, Rowland DG. Ruptured myocardial abscess causing left ventricle to pulmonary artery communication in an infant with community-associated methicillin-resistant Staphylococcus aureus endocarditis. Arch Pathol Lab Med 2011;135:1057-60. [Crossref] [PubMed]
- Valdis M, Gelinas J, Guo L. Invasive Methicillin-Resistant Staphylococcus Aureus Endocarditis Resulting in Right Ventricular Pseudoaneurysm and Fistulation to Previous Bypass Graft. Ann Thorac Surg 2015;100:e5-7. [Crossref] [PubMed]
- Jeannon JP, Orabi A, Manganaris A, et al. Methicillin Resistant Staphylococcus Aureus Infection as a causative agent of fistula formation following total laryngectomy for advanced head & neck cancer. Head Neck Oncol 2010;2:14. [Crossref] [PubMed]
- Alohali AF, Abu-Daff S, Alao K, et al. Ventilator Management of Bronchopleural Fistula Secondary to Methicillin-Resistant Staphylococcus aureus Necrotizing Pneumonia in a Pregnant Patient with Systemic Lupus Erythematosus. Case Rep Med 2017;2017. [Crossref] [PubMed]
- Boyd DP. Bronchobiliary and bronchopleural fistulas. Ann Thorac Surg 1977;24:481-7. [Crossref] [PubMed]
- Dai H, Cui D, Li D, et al. Hepatic abscess with hepatobronchial fistula following percutaneous radiofrequency ablation for hepatocellular carcinoma: A case report. Oncol Lett 2015;9:2289-92. [Crossref] [PubMed]
- Goh CX, Pua U. Hepatobronchial fistula following multiple-session doxorubicin-eluting bead chemoembolization in a large hepatocellular carcinoma. J Vasc Interv Radiol 2014;25:150-2. [Crossref] [PubMed]
- Moawad F, Truesdell A, Mulhall B. A. "fishy" cough: hepatobronchial fistula due to a pyogenic liver abscess. N Z Med J 2006;119:U1906. [PubMed]
- Ala A, Safar-Aly H, Millar A. Metallic cough and pyogenic liver abscess. Eur J Gastroenterol Hepatol 2001;13:967-9. [Crossref] [PubMed]
- Mazziotti S, Gaeta M, Blandino A, et al. Hepatobronchial fistula due to transphrenic migration of hepatic echinococcosis: MR demonstration. Abdom Imaging 2000;25:497-9. [Crossref] [PubMed]
- Yasufuku M, Yamamoto H, Yamamoto H, et al. Hepatobronchial fistula caused by intrahepatic bile duct carcinoma. Nihon Geka Gakkai Zasshi 1994;95:712-5. [PubMed]
- von Birgelen C, von Schönfeld J, Görge G, et al. Amebic liver abscess with hepatobronchial fistula]. Dtsch Med Wochenschr 1994;119:1034-8. [Crossref] [PubMed]
- Stables GI, Irving HC, Simmons AV, et al. Case report: hepatobronchial fistula complicating amoebiasis, treated by percutaneous catheter drainage. Clin Radiol 1991;44:354-6. [Crossref] [PubMed]
- Nesper M, McGahan JP. Hepatobronchial fistula with percutaneous pyogenic abscess drainage of the liver. Gastrointest Radiol 1985;10:129-31. [Crossref] [PubMed]
- Borrie J, Shaw JH. Hepatobronchial fistula caused by hydatid disease. The Dunedin experience 1952-79. Thorax 1981;36:25-8. [Crossref] [PubMed]
- Okuda K, Kanda Y, Fukuyama Y, et al. Spontaneous hepatobronchial communications preceding pyothorax in a patient with suspected liver abscess. A case report. Gastroenterology 1973;65:124-9. [PubMed]
- Goijman I. Amebic abscess of the liver; hepatobronchial fistula. Sem Med 1952;101:628-9. [PubMed]
- Michels AG, Van Ordstrand HS, Collins EN. Amoebic hepatic abscess with bronchohepatic fistula; report of a case. Cleve Clin Q 1949;16:142-7. [Crossref] [PubMed]
- McDougal LK, Steward CD, Killgore GE, et al. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol 2003;41:5113-20. [Crossref] [PubMed]