Does age over 80 years have to be a contraindication for lung cancer surgery—a nationwide database study
Introduction
Age is an important risk factor for morbidity and mortality after lung resection for non-small cell lung cancer (NSCLC), and therefore, curative lung cancer (LC) surgery is less often proposed to older patients (1). In the last decade, life expectancy has increased, and in 80-year-old patients it is around 8.2 years for men and 9.6 years for women (2). Predictions estimated that in 2050, more than 20% of the world’s population will be 80 years old or older (3). Today, LC is the second leading cause of death in octogenarians after heart disease but remains the leading cause of death by cancer in the US, with around 174,000 deaths of octogenarians in 2012 (4). In elderly patients, the probability of developing LC is 6.4% for men (1 in 16 men) and 4.8% in women (1 in 21 women) (4).
According to the 2013 guidelines of the American College of Chest Physicians, surgical resection remains the best treatment for primary LC (5). In the last decade, improvements have been made in the quality of preoperative assessments and postoperative care, and these have led to a decrease in postoperative mortality following LC surgery (6). Today, more and more octogenarians present localized LC that is eligible for surgical resection. Despite an increased number of these patients, there are few reports in the literature and most of those that have been published have fewer than 100 patients, and no results from large national database analyses are available (7-12).
The objective of the present study was to determine in-hospital mortality (IHM) in patients aged 80 years and older undergoing LC surgery and to determine whether age is the most important predictor of IHM within a large national cohort of patients operated on for LC.
Methods
Data source and study population
Data from patients who underwent pulmonary resection for LC in France from January 2005 to December 2015 were collected from the national administrative database. This national database, called PMSI for “Programme de Médicalisation des Systèmes d’Information”, was inspired by the US Medicare system. The reliability and validity of PMSI data have already been assessed (13). Routinely collected medical information includes the principal diagnosis, secondary diagnoses and procedures performed. Diagnoses identified during the hospital stay are coded according to the International Classification of Diseases, 10th revision (ICD-10) (14). We selected patients with a diagnosis of primary LC coded as the principal discharge diagnosis (all codes C34). Procedures were coded according to the CCAM (Classification Commune des Actes Médicaux). All patients were provided with information regarding the collection of medical data relative to their hospitalization. For all patients, LC was pathologically proven according to the 2004 World Health Organization classification of LC (15). Surgery-related variables included the surgical approach [thoracotomy, video-assisted thoracic surgery (VATS)], the type of resection [sublobar resection (wedge resection and anatomical segmentectomy), lobectomy, bilobectomy and pneumonectomy], bronchoplasty, and the extent of the pulmonary resection (to the chest wall, the left atrium, the carina, the diaphragm, and the superior vena cava). The study period was 2005 to 2015.
Patients’ characteristics
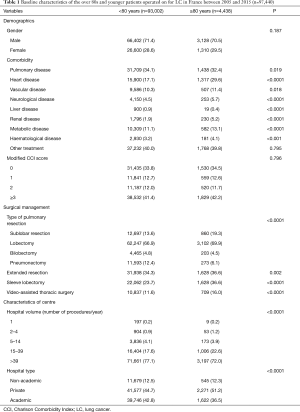
Baseline demographics included age and gender. From the national administrative database, we included the following comorbidities: pulmonary disease (chronic bronchitis, emphysema), alcohol abuse, liver disease, cerebrovascular events, neurological diseases (hemiplegia or paraplegia), dementia, diabetes mellitus with and without complications, renal disease, coagulopathy, leukaemia, lymphoma, ulcerative disease, history of malignant disease, obesity, other therapies (preoperative chemotherapy, steroids). We also calculated the modified Charlson Comorbidity Index (CCI) (16).
Outcome measurements
IHM was defined as any patient who died in hospital (including patients transferred to other departments or hospitals) within the first 30 days after the operation and those who died later during the same hospitalization.
Statistical analyses
To determine independent factors for in-hospital death, we first performed univariate analysis with the χ2 test for binary and categorical variables and a t-test for continuous variables. Variables with a level of significance of 0.1 or less in the univariate analysis were included in the multivariate logistic regression. Predicted mortality was calculated from a multiple regression model including age, sex, comorbidities, period of time, and hospital characteristics. Interaction effects were sought for all variables included in the model. Hospital volume was extrapolated according to the median number of procedures performed per center per year, and was a ranked variable grouped into five quintiles (1, 2–4, 5–14, 15–39 and >39). For the regression analysis, hospital volume and period of time were continuous variables and the modified CCI score and type of pulmonary resection were ranked variables. Other variables were binary. Log-linearity was tested for age, period of time and hospital volume.
Our decision tree was built using the hierarchy of the weight of each predictive factor for IHM determined by logistic regression.
Validation
The area under the receiver operating characteristic curve, the index-corrected Dxy correlation, and the R2 value were used to measure the discriminatory ability of the model (17,18). The reliability of the model was estimated by the relationship between the predicted probability and the observed outcome. Calibration by plotting predicted against observed probability can estimate intercepts and slopes of curves to quantify overfitting (18). Well-calibrated models have a slope of 1, whereas models that provide overly extreme predictions have a slope of less than 1: low predicted probabilities are too low and high predicted probabilities are too high.
The internal validation of the model was assessed with bootstrap resampling techniques (18). A model in the bootstrap sample (test set) is derived and applied to the original sample without change. The discriminatory and reliability index from the bootstrap sample minus the index computed on the original sample is an estimate of optimism. This process is repeated for 100 bootstrap replications to obtain an average optimism, which is subtracted from the apparent accuracy of the final model’s (corrected index) fit to obtain the overfitting-corrected estimate (18).
We used a regression tree to approximate the model to make it easier to understand, using the model’s predicted probability as the dependent variable. For each terminal node, we showed the distribution of the model’s predicted probabilities (18). Discrete variables were expressed as numbers with percentages, and continuous variables as means and standard deviations. STATA 14 statistical software (StataCorp, College Station, Tex) and R statistical software, for which we used Harrell’s Design library (http://www.r-project.org), were used for the analyses.
Results
Patients’ characteristics
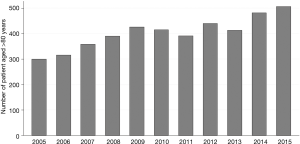
From January 2005 to December 2015, 97,440 patients, including 4,438 patients aged 80 years old or older, were operated on for LC in France. There was a trend towards an increase in the annual number of patients operated on in France from 2005 to 2015 (P=0.43) (Figure 1). Patients’ characteristics are reported in Table 1. Among the over 80s, there was a significantly greater proportion of patients with comorbidities: kidney disease, neurological disease, metabolic disease and haematological disease (Table 1). There was no significant difference between groups regarding the gender or the modified CCI score (Table 1).
Full table
Management
As compared to younger patients, patients over 80 years old had more sublobar resections and lobectomies (19.3% vs. 13.6% and 69.9% vs. 66.9%, respectively), and fewer pneumonectomy (6.1% vs. 12.4%) (P<0.0001) (Table 1). The over 80s more often underwent surgery in medium-volume centres (15 to 39 LC surgeries a year) than did younger patients (22.6% vs. 17.6%) and less often in high-volume centres (>39 LC surgeries a year) (72.0% vs. 77.1%) (P<0.0001) (Table 1). Moreover, a significantly higher proportion of patients over 80 than younger patients were operated on in private institutions (51.2% vs. 44.7%; P<0.0001) (Table 1).
IHM and prognostic factors
After surgery, crude IHM was 3.73% (n=3,639), and was higher for patients over 80 than for younger patients: 7.77% (n=345) vs. 3.54% (n=3,294), respectively (P<0.0001). For the over 80s, IHM was 5.9% (n=51) for sublobar resection, 7.3% (n=226) for lobectomy, 10.8% (n=22) for bilobectomy and 16.8% (n=46) for pneumonectomy.
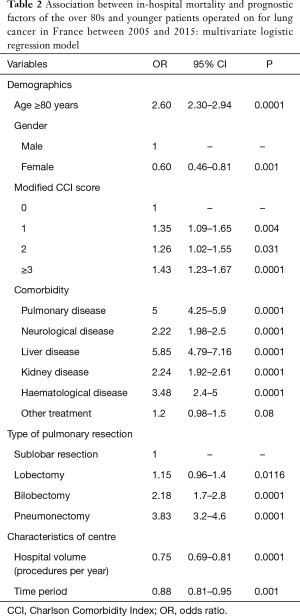
In the logistic model, IHM was significantly linked to an age of 80 years or older, a CCI score ≥3 and a comorbidities of liver disease, pulmonary disease, haematological disease, kidney disease, and neurological disease (Table 2).
Full table
IHM was also significantly linked to the type of pulmonary resection: the adjusted OR was 1.15 (95% CI: 0.96–1.4; P=0.0116) for lobectomy, 2.18 for bilobectomy (95% CI: 1.7–2.8; P=0.0001) and 3.83 (95% CI: 3.2–4.6; P=0.0001) for pneumonectomy compared to limited resection (Table 2).
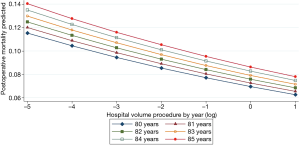
IHM was also linked to hospital volume and the time period (from 2005 to 2015) with ORs of 0.75 (95% CI: 0.69–0.81; P=0.0001) and 0.88 (95% CI: 0.81–0.95; P=0.001), respectively (Table 2). There was a linear decrease in the predicted IHM according to hospital volume whatever the age over 80 years old (Figure 2).
Validation
The performance of the model for the training set and its ability to predict IHM for the test set are reported in Table 3. The index-corrected Dxy correlation and R2 values were no different between the training set and test set (Table 3). The slope-correction factor was 0.99 (Table 3).
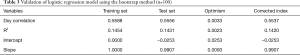
Full table
Regression tree
The regression tree was used to assess the predicted IHM after lung resection regarding the patients’ comorbidities (Figure 3). It showed that for any patient, IHM was strongly linked to a comorbidity of pulmonary and liver disease. In such patients who needed pneumonectomy, predicted IHM was 49% (Figure 3).
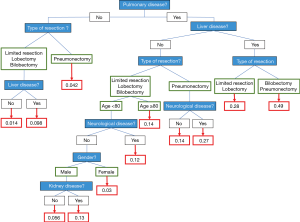
It also showed that for any patient, including the over 80s, with no pulmonary comorbidities, predicted IHM was 4.2% after pneumonectomy, and 1.4% after lesser resection in patients without liver disease. In our regression tree, age was taken into account only for patients with a comorbidity of pulmonary disease (but not liver disease) and undergoing pulmonary resection (less invasive than pneumonectomy). IHM reached 14% for the over 80s and was lower for younger patients (Figure 3). Indeed, age had a lower weight in predicted IHM than did a comorbidity of pulmonary or liver disease, or the type of pulmonary resection.
Discussion
It is difficult to select patients over 80 years old for surgical management. Few articles on this population are available and in most clinical trials on medical management less than 25% of patients were older than 65 years old (19). Moreover, published evidence suggests that elderly patients are denied potentially beneficial treatment and participation in clinical trials solely because of chronological age and because of the physician’s perception (20-22). In a recent analysis of the SEER database, in 47,912 octogenarians managed for LC, only 5% underwent surgery for localized LC and 47% received neither surgery nor radiotherapy (20). Frequent reasons for withholding therapy from elderly patients were the fear of toxicity and of increased morbidity and mortality, which could be a genuine, but not always justified, concern (20), thus, raising the question of how to select patients over 80 years old for surgical resection of localized LC.
Patients’ characteristics
In our population, as in previous publications, the proportion of women among the over 80s was around 30% (7,8,10,12). A comorbidity of pulmonary disease was present in 32.4%, which is similar to the 37.2% reported by Port et al. (11), but lower than the 47.1% of Matsuoka et al. (12). We also found more patients with liver, metabolic and haematological comorbidities than in other studies. These comorbidities came to light because of the large number of patients in our study. These findings will lead to clearer criteria for the selection of patients fit enough for surgical resection.
IHM
IHM for patients over 80 years old was 7.77%, which is higher than the 1.15% to 6.3% reported over the last decade (7-12). This high IHM in our study could be explained first by the high proportion of over 80-year-old undergoing surgery in middle-volume centres, which was a predictor of IHM in multivariate analysis, and second, because around 11% of patients underwent pneumonectomy or bilobectomy, which is dramatically higher than in other studies dealing with octogenarians (7-12). Moreover, as described by von Meyenfeldt et al. in a meta-analysis of 11 studies, our study highlighted that IHM was strongly linked to the annual hospital volume of pulmonary resections (23), and the fact that around 23% of patients over 80 years old with LC in France underwent surgery in middle-volume hospitals could explain this high IHM for the whole database.
Type of pulmonary resection and surgical management
Our study showed high IHM for bilobectomy and pneumonectomy, at 10.8% and 16.8%, respectively; which is dramatically higher than the 5.9% for sublobar resection and 7.3% for lobectomy. However, this IHM for pneumonectomy was lower than the 23.3% reported by Dell’Amore et al. in a series of 319 patients, including 83 octogenarians (24). In fact, the more extensive the pulmonary resection, from sublobar resection to pneumonectomy, the greater the increase in IHM for the over 80s. In multivariate analysis, the odds ratio for IHM was 2.18 for bilobectomy and 3.83 for pneumonectomy as compared to sublobar resection; however, lobectomy was not linked to an increase in IHM. Similar results have already been reported, not only in octogenarians but also in the general population (24,25). These findings strongly encourage thoracic surgeons not to perform resections larger than lobectomy in patients over 80 years old, and to avoid bilobectomy or pneumonectomy. The VATS approach was significantly linked to IHM in univariate but not in multivariate analysis, as were the extent of the resection and sleeve resections. The number of VATS was very low in our study, because VATS really emerged in 2014 in France, and we can expect that the IHM for the over 80s will decrease in the next few years because of the increased use of minimally invasive approaches for LC.
Regression tree
Our regression tree was built using the weight of each preoperative and preoperative variable to predict IHM. Comorbidities of pulmonary and liver disease seemed to be major preoperative variables to predict IHM after LC surgery, and were stronger than age. Indeed, age over 80 years old increased IHM by a factor of 2.6, but the weight of this variable was far less than that of comorbidities such as lung or liver disease, with respective odds ratios of 5 and 5.85. Preoperative interstitial lung disease has been reported to increase postoperative mortality (26). Moreover, publications dealing with IHM after major surgeries, such as cardiac or colorectal surgery, highlighted the dramatic impact of liver dysfunction on IHM (27,28). Indeed, liver dysfunction leads to an increased transfusion requirement, decreased platelet count, increased prothrombin time and finally to decompensated liver disease (27).
In our opinion, old age is not a major concern for the surgical management of LC. As in other patients, the preoperative assessment remains the most important factor in decisions for LC surgery. In our study, a history of heart disease was not a predictor of IHM in multivariate analysis, probably because patients unfit for surgery because of heart disease were denied surgical management. Moreover, heart disease has often been associated with postoperative complications after LC surgery (29). Therefore, a systematic evaluation of cardiac and pulmonary function, as recommended by the guidelines of different thoracic societies, is essential to determine which patients are fit enough for surgical resection (29,30). When performed by board certified surgeons in centres with hospital volumes over 39 procedures a year, surgical management of the over 80s, by lobar or sublobar resections, seems to be linked to an acceptable IHM ranging from 1% to 6% (7-12). This is why decision-making for the management of NSCLC in the over 80s needs the expertise of certified physicians, oncologists and thoracic surgeons so as not to deny surgery for patients only because of their age. Therefore, patients over 80 years old without significant liver, pulmonary and cardiovascular comorbidities should be referred for surgery if lobar or sublobar resection can be performed.
Limitations
Our study has various limitations. Given the reliance on ICD-10 codes to select patients and ascertain outcomes, there was a potential for misclassification- or underdetection-related bias especially for comorbidities. Coding practices vary significantly among institutions. Nevertheless, coding quality is checked by medical information professionals in each hospital to correct diagnoses and improve the recording of comorbidity. No details were available concerning disease stage, postoperative complications, and data for 90-day mortality.
However, the demographic characteristics, risk factors, and outcomes of the present study population were very similar to those in previous French studies from the Epithor database (6).
Unlike the Epithor database, the French Administrative database does not record LC information, such as the stage of the disease, preoperative forced expiratory volume, or the American Society of Anesthesiologists score.
Despite these limitations, the strength of our results is related to the large size of our sample (97,440 patients including 4,438 over 80 years old), with national recruitment.
Conclusions
The variable of age had a lower weight in the predicted IHM than did comorbidities of pulmonary and liver disease, or the type of pulmonary resection. A systematic preoperative assessment to evaluate pulmonary, cardiac, liver and renal function precisely is mandatory in the over 80s. Lobectomy or less invasive resections are not linked to an increased risk of postoperative death as compared to bilobectomy or pneumonectomy, which are not acceptable for the management of the over 80s. Therefore, patients over 80 years old without liver, renal, pulmonary and/or cardiovascular comorbidities should be referred to surgery if lobectomy or sublobar resection can be performed.
Acknowledgements
The authors would like to thank Philip Bastable and Suzanne Rankin (Dijon University Hospital) for revising the manuscript. This work was supported by the Multi-organization Cancer Institute (Institut thématique multi-organismes cancer: ITMO Cancer), the Public Health Research Institute (Institut de Recherche en Santé Publique: IRESP) and the French National Institute of Health and Medical Research (Institut national de la santé et de la recherche médicale: INSERM) in the context of the 2014–2019 cancer plan.
Funding: This study was funded by the Research Department of Dijon University Hospital.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Every patient operated in France receive the information which confirm that all medical data concerning their hospitalization will be collected into the French administrative database and could be used for research. If they don’t agree, the information’s relatives to their hospitalization won’t be collected. There is only an information and no signed consent, as described in the French law, there’s no need for ethics committee.
References
- Bernard A, Rivera C, Pagès PB, et al. Risk model of in-hospital mortality after pulmonary resection for cancer: a national database of the French Society of Thoracic and Cardiovascular Surgery (Epithor). J Thorac Cardiovasc Surg. 2011;141:449-58. [Crossref] [PubMed]
- . Available online: http://www.ssa.gov/OACT/STATS/table4c6.htmlSocial Security Administration. 2010.
- United Nations Department of Economic and Social Affairs population Division. World population ageing: 1950e2050. Available online: http://www.un.org/esa/population/publications/worldageing19502050
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Morgant MC, Pagès PB, Orsini B, et al. Time trends in surgery for lung cancer in France from 2005 to 2012: a nationwide study. Eur Respir J 2015;46:1131-9. [Crossref] [PubMed]
- Dominguez-Ventura A, Allen MS, Cassivi SD, et al. Lung cancer in octogenarians: factors affecting morbidity and mortality after pulmonary resection. Ann Thorac Surg 2006;82:1175-9. [Crossref] [PubMed]
- Brokx HAP, Visser O, Postmus PE, et al. Surgical treatment for octogenarians with lung cancer: results from a population-based series of 124 patients. J Thorac Oncol 2007;2:1013-7. [Crossref] [PubMed]
- Suemitsu R, Yamaguchi M, Takeo S, et al. Favorable surgical results for patients with nonsmall cell lung cancer over 80 years old: a multicenter survey. Ann Thorac Cardiovasc Surg 2008;14:154-60. [PubMed]
- Okami J, Higashiyama M, Asamura H, et al. Pulmonary resection in patients aged 80 years or over with clinical stage I non-small cell lung cancer: prognostic factors for overall survival and risk factors for postoperative complications. J Thorac Oncol 2009;4:1247-53. [Crossref] [PubMed]
- Port JL, Mirza FM, Lee PC, et al. Lobectomy in octogenarians with non-small cell lung cancer: ramifications of increasing life expectancy and the benefits of minimally invasive surgery. Ann Thorac Surg 2011;92:1951-7. [Crossref] [PubMed]
- Matsuoka K, Ueda M, Miyamoto Y. Risk factor for respiratory death after lung cancer surgery in octogenarians. Asian Cardiovasc Thorac Ann 2015;23:1044-9. [Crossref] [PubMed]
- Iezzoni LI. Assessing quality using administrative data. Ann Intern Med 1997;127:666-74. [Crossref] [PubMed]
- International Statistical Classification of Diseases and Related Health Problems 10th Revision. Available online: http://apps.who.int/classifications/icd10/browse/2016/en
- Travis WD, Brambilla E, Müller-Hermelink HK, et al. Pathology and Genetics: Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC, 2004.
- Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245-51. [Crossref] [PubMed]
- Hilbe JM. Logistic Regression Models. Boca Raton, FL: Chapman & Hall Book, 2009:1-637.
- Harrell FE. Regression modeling strategies with applications to linear models, logistic regression and survival analysis. New York: Springer, 2001:1-330.
- Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 1999;341:2061-7. [Crossref] [PubMed]
- Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol 2007;25:5570-7. [Crossref] [PubMed]
- Taylor KM, Feldstein ML, Skeel RT, et al. Fundamental dilemmas of the randomized clinical trial process: results of a survey of the 1,737 Eastern Cooperative Oncology Group investigators. J Clin Oncol 1994;12:1796-805. [Crossref] [PubMed]
- Sawada S, Komori E, Nogami N, et al. Advanced age is not correlated with either shorerm or long-term postoperative results in lung cancer patients in good clinical condition. Chest 2005;128:1557-63. [Crossref] [PubMed]
- von Meyenfeldt EM, Gooiker GA, van Gijn W, et al. The relationship between volume or surgeon specialty and outcome in the surgical treatment of lung cancer: a systematic review and meta-analysis. J Thorac Oncol 2012;7:1170-8. [Crossref] [PubMed]
- Dell’Amore A, Monteverde M, Martucci N, et al. Early and long-term results of pulmonary resection for non-small-cell lung cancer in patients over 75 years of age: a multi-institutional study. Interact Cardiovasc Thorac Surg 2013;16:250-6. [Crossref] [PubMed]
- Voltolini L, Bongiolatti S, Luzzi L, et al. Impact of interstitial lung disease on short-term and long-term survival of patients undergoing surgery for non-small-cell lung cancer: analysis of risk factors. Eur J Cardiothorac Surg 2013;43:e17-23. [Crossref] [PubMed]
- Rapicetta C, Tenconi S, Voltolini L, et al. Impact of lobectomy for non-small-cell lung cancer on respiratory function in octogenarian patients with mild to moderate chronic obstructive pulmonary disease. Eur J Cardiothorac Surg 2011;39:555-9. [Crossref] [PubMed]
- Dimarakis I, Grant S, Corless R, et al. Impact of hepatic cirrhosis on outcome in adult cardiac surgery. Thorac Cardiovasc Surg 2015;63:58-66. [PubMed]
- Pallis AG, Gridelli C, van Meerbeeck JP, et al. EORTC Elderly Task Force and Lung Cancer Group and International Society for Geriatric Oncology (SIOG) experts’ opinion for the treatment of non-small-cell lung cancer in an elderly population. Ann Oncol 2010;21:692-706. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-90S.
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17-41. [Crossref] [PubMed]


