Impact of previous head and neck cancer on postoperative complications after surgical resection for lung cancer: a case-control study
Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related death in Western countries. Lung resection (LR) is the main treatment for early NSCLC. Postoperative mortality after LR has decreased over the past decade due to improvements in surgical and anesthesia techniques (1,2), as well as better postoperative management (3-5). Known risk factors for postoperative complications after LR include anesthesia duration, preoperative lung function, older age, cardiovascular comorbidity, smoking status, preoperative chemotherapy, intraoperative bleeding, and type of surgery (6-13).
Head and neck cancer (HNC) is the most frequent primitive cancer associated with lung cancer (14,15). It may be synchronous or metachronous. The most frequent second malignant tumor among patients previously treated for HNC is lung cancer (14). Swallowing disorders are common after HNC treatment and are reported in up to 88% of patients depending on the tumor location, tumor size, and treatment (16,17). These problems mainly appear after radiation or partial laryngeal resection. Swallowing disorders may favor aspiration pneumonia (18,19). Few studies have evaluated the risk of postoperative complications after LR among patients who have previously been treated for HNC.
The objective of this retrospective, case-control study was to investigate the risk factors for postoperative complications in patients with previous HNC undergoing LR for lung cancer.
Methods
This retrospective, case-control study was carried out between January 2006 and May 2012 in a single academic thoracic surgery center at the Hôpital Européen Georges Pompidou, Paris, France.
Study population
Patients were selected from the hospital clinical database using three search criteria: (I) primary malignant lung tumor [ICD10 code (C34)]; (II) one of the following surgical procedures (pulmonary lobectomy, or pulmonary bilobectomy, or pneumonectomy, or wedge resection); and (III) HNC (ICD10 codes C00–C14, C31, C32). A specialist of HNC cancer (OL) checked that HNC had been diagnosed and treated before LR and reviewed each patient’s file. The head and neck surgeon clinically assessed swallowing function blinded for postoperative complications. Cases of synchronous cancer and HNC diagnosed after lung cancer surgery were excluded. A control group was selected of patients who fulfilled criteria 1 and 2 above, but did not have previous HNC.
The study population was divided into two groups. Group 1 (Gp1) consisted of control patients undergoing LR without previous HNC, and Group 2 (Gp2) consisted of patients undergoing LR after previous HNC. In Gp1, two patients were randomly selected for every patient in Gp2. Patients were matched 2/1 for the following variables: age at the time of thoracic surgery, gender, preoperative FEV1, smoking status, and year of surgery.
Postoperative management
Antibiotic prophylaxis was administered based on national guidelines. Pain was managed with systemic opioids released by a patient-controlled administration system. Epidural analgesia or intercostal blocks were used at the discretion of the anesthesiology team. Physiotherapy was started at least twice-daily commencing the day after surgery. All patients were maintained in a semi-recumbent position and were confined to a chair on the first day after surgery. Postoperative pain levels were recorded using a visual analogue scale. The chest tube was removed if there was no bubbling and fluid loss was <150 mL/day. A chest X-ray was performed and examined daily.
Data collection
The following data on the previous HNC were collected from the patients’ medical records: date of diagnosis, histology, TNM stage, localization, surgical procedure, associated treatments (radiotherapy or chemotherapy), and presence of tracheostomy. For the lung cancer the following data were recorded: histology, TNM stage, preoperative treatments, type of LR (pneumonectomy, lobectomy or wedge resection), duration of surgery, resection side, transfusion, Mallampati score. The maximum pain level during the first 24 h post-surgery was recorded.
Outcome
The primary endpoint was the occurrence of one of the following events during the first 30 days post LR: death, shock (use of a vasopressor agent), need for mechanical ventilation (invasive or non-invasive), or postoperative pneumonia (POP).
POP was diagnosed using the following criteria: (I) fever >38 °C, or hypothermia <36 °C; (II) new or change in pulmonary infiltrate on chest X-ray; and (III) one of the following additional criteria: purulent sputum or leukocytosis >109/L or <2.5×109/L. Two investigators (BP and GM) blinded to the presence or absence of previous HNC assessed all diagnoses of POP to avoid any potential bias.
Statistical analysis
Statistical analysis was performed using STATA®, v.11 for Windows (StataCorp., Texas, USA). Data are summarized as the mean ± standard deviation for quantitative variables, or number and percentage for categorical variables. Postoperative outcome was studied by univariate analysis using the Chi2 or Fisher’s exact test to compare quantitative variables and Student’s t-test or Kruskall Wallis test to compare continuous variables.
Variables yielding P values <0.1 in univariate analysis were included in a forward multivariate logistic regression analysis taking collinearity into account, to identify the factors that were predictive for postoperative outcome/event. Likelihood ratio statistics were used as a criterion for selection in a backward stepwise procedure. All tests were two-tailed and P<0.05 was considered statistically significant.
Results
Study population
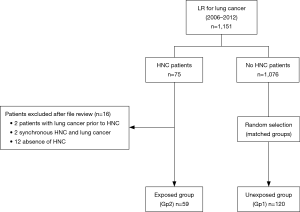
From January 2006 to May 2012, 1,151 patients underwent LR for lung cancer. Among these patients, 75 were identified as having previous HNC. Sixteen patients were excluded after a review of their files because two patients had lung cancer prior to HNC, two had synchronous HNC and lung cancer, and 12 had no HNC. The remaining 59 patients defined the exposed group and were included in Gp2 (Figure 1).
A total of 1,076 patients without previous HNC underwent LR for lung cancer and 120 of these were matched and comprised the unexposed group (Gp1) (Figure 1).
This study was conducted in accordance with the amended Declaration of Helsinki. Local institutional review board (N°3001 on the 10th May, 2011) and Commission Nationale Informatique et Liberté (CNIL) (N°1922081; 02/02/2016) approved the protocol.
Clinical characteristics and management of lung cancer
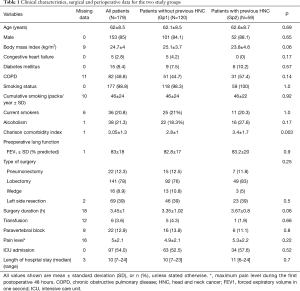
The demographic and clinical characteristics of the study population are shown in Tables 1,2. Most patients (85%) were male, and mean age at the time of surgery was 62±8 years. The Charlson score was significantly higher in Gp2 (3.4±1.7 vs. 2.8±1; P=0.003) and there were more squamous cell carcinomas in Gp2 than in the control group (Gp1) (57.6% vs. 31.6%, P=0.003%) (Table 2).
Full table

Full table
In Gp2, the median time between HNC treatment and lung cancer surgery was 80 months. Laryngeal cancer was the most frequent localization of HNC (n=24, 40.6%). Most patients underwent surgical treatment (n=31, 62%) and 27 (50.0%) received chemotherapy and/or radiotherapy. Among these 59 patients, 41 were evaluated for swallowing disorders before LR and 6 (14%) had a major swallowing disorder (Table S1).

Full table
All pulmonary resections were included. Concerning extended resections, one patient in Gp1 and two patients in Gp2 underwent intrapericardial pneumonectomies, five LRs in Gp1 and one patient in Gp2 were associated with chest wall resection, one patient in Gp2 had diaphragm resection and one patient in Gp1 was treated with left atrium resection.
Thoracic surgery management did not differ between the two groups (Table 1). Thirty-four patients in Gp2 (57%) were cared for in the intensive care unit (ICU) vs. 63 patients (52%) in Gp1. Median length of stay in the ICU after surgery was significantly higher in Gp2 than in Gp1 patients [6 days (3–16) vs. 4 days (1–11), P=0.004]. There was no difference in the total length of hospital stay between the two groups [10 days (7–23) for Gp1 vs. 11 days (6–24) for Gp2, P=0.7].
Primary endpoint
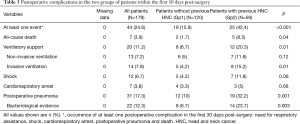
In the overall population, 44 patients (24.6%) experienced at least one postoperative event during the first 30 days post-surgery (Table 3). Among them, 31 patients (17.3%) had POP, which was confirmed bacteriologically in 22 cases (71%). Postoperative complications [42.4% (95% CI, 29.4–55.4%) vs. 15.8% (95% CI, 9.2–22.5%), P<0.001], POP (32.2% vs. 10%, P=0.001), and all-cause mortality (8.5% vs. 1.7%, P=0.04) were significantly more common in HNC patients (Gp2) when compared to Gp1. The documented bacterial species in the HNC patients with pneumonia were all enterobacterias.
Full table
Type and treatment received for HNC according to the occurrence of postoperative complication
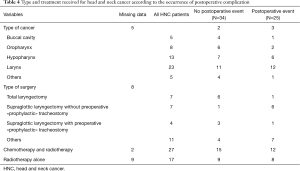
In the group of patients with HNC, 7 were treated by total laryngectomy, 1 of whom experienced a postoperative complication. Eleven patients were treated by supraglottic partial laryngectomy. Among them, a postoperative complication occurred in 7 patients; a tracheostomy was performed during thoracic surgery (i.e., “prophylactic”) in only one patient (Table 4). Six patients were classified as high risk for swallowing disorders: four of these experienced a serious postoperative event (Table S1).
Full table
Risk factors for postoperative complications
Multivariate analysis revealed the following risk factors to be independently associated with a high risk of complications in the first 30 days post-surgery: history of HNC [OR =4.24 (95% CI, 1.84–9.74)], male gender [OR =8.99 (95% CI, 1.05–76.78)], cumulative smoking [OR =1.02 for an increase of 1 pack-year (95% CI, 1.01–1.04)], Charlson comorbidity index [OR =1.45 (95% CI, 1.07–1.96)] (Table 5).

Full table
Discussion
The present study assessed the incidence of serious postoperative complications in 179 patients undergoing lung cancer surgery with or without previous HNC. The data suggest that patients with previous HNC have an increased risk of postoperative complications including POP and death. Previous HNC was identified as an independent risk factor for severe postoperative complications after LR. Other independent risk factors for postoperative complications included cumulative smoking status, male gender, and high Charlson comorbidity score.
The characteristics of our study population are consistent with previous LR cohorts (5). Patients with a high Charlson comorbidity index and cumulative smoking of over 40 packs/year usually present with cardiovascular comorbidities (5). The type of surgery was also similar to that reported in the literature although the incidence of pneumonectomy was higher than reported (20). The incidence of serious postoperative events was higher than that reported elsewhere. Nevertheless, postoperative mortality in our study remained within the usual range, from 1.3–5.2% (5,20). The higher complication rate including higher POP (21) could be explained by the selection of high-risk patients, mostly HNC patients, and the large proportion of patients undergoing pneumonectomy. The postoperative events in the HNC group were similar in type to those in Gp1.
Our study confirms several risk factors for severe postoperative complications, namely male gender (21), and Charlson comorbidity index (22,23). Smoking history is a known risk factor for severe postoperative complications after LR (24). However, we found no protective effect of smoking cessation before surgery, as reported recently by Rodriguez et al. (25).
Only a few studies have reported the outcome of patients with previous HNC who undergo LR. Massard et al. carried out an observational study of 114 patients with previous HNC undergoing LR (26). They reported a mortality rate of 3.5% and a POP rate of 17.6%. More recently, Herrera et al. reported a larger exposed/unexposed study and found an increase in POP, mainly after partial laryngeal surgery (27).
Swallowing disorders seem to be the main problem in HNC patients. Four out of six of our patients who were evaluated as high-risk for swallowing before LR developed POP (Table S1). All had previously been treated by partial laryngeal resection. Furthermore, although POP was not documented systematically by microbiological sampling, the documented bacteria in HNC patients with POP were all enterobacteria species, supporting the hypothesis that an aspiration mechanism is involved. It has recently been suggested that up to 25% of patients will develop aspiration pneumonia within 5 years after receiving chemo-radiotherapy for HNC (28). These data suggest that a systematic evaluation of preoperative swallowing disorders could help in identifying high-risk patients for postoperative complications in whom preventive strategies could be proposed.
Even if our results and those of Herrera et al. (27) support the hypothesis that swallowing disorders may increase the risk of POP in the early postoperative phase, the benefit of preventive tracheostomy in HNC patients has not been validated. However, temporary “prophylactic” tracheostomy during LR surgery could facilitate the clearance and aspiration of bronchial secretions and help to introduce respiratory assistance if necessary. Although many centers have adopted this strategy for some patients, there is no consensus regarding the treatment of these high-risk patients. As we noted, among the 11 HNC patients treated by supraglottic partial laryngectomy, seven experienced at least one serious postoperative complication. However, a prophylactic tracheostomy was performed during LR in four of these patients and only one had a serious postoperative complication. Moreover, patients treated by total laryngectomy cannot have swallowing disorders. Only 1 out of 7 patients treated by total laryngectomy had a postoperative complication. After exclusion of these 7 patients, the results of the multivariate analysis remained unchanged.
The main strength of our work is its exposed/non-exposed study design with groups matched for most of the usual risk factors for postoperative complications. However, our study has several limitations. It was a monocenter analysis with only a small number of patients with previous HNC. Because of its retrospective design, some data were also missing, especially data regarding the preoperative swallowing evaluation. Finally, not all POPs were documented by a positive bacterial culture from a respiratory sample. However, we used a well-accepted definition of nosocomial pneumonia and all events were independently assessed by two investigators blinded to the presence or absence of previous HNC.
Conclusions
The present study confirms that previous HNC is a major risk factor for serious postoperative complications in patients undergoing LR for lung cancer. Male gender, high Charlson comorbidity index, and smoking status were independently associated with the occurrence of postoperative complications. This increased risk of complications may be related to swallowing disorders, a frequent complication after HNC surgery. Our data support the systematic preoperative clinical assessment of swallowing disorders to identify patients at risk. The risk:benefit ratio of a temporary “prophylactic” tracheostomy should be evaluated.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was conducted in accordance with the amended Declaration of Helsinki. Local institutional review board (N°3001 on the 10th May, 2011) and Commission Nationale Informatique et Liberté (CNIL) (N°1922081; 02/02/2016) approved the protocol.
References
- Riquet M, Berna P, Fabre E, et al. Evolving characteristics of lung cancer: a surgical appraisal. Eur J Cardiothorac Surg 2012;41:1019-24. [Crossref] [PubMed]
- Joshi GP, Bonnet F, Shah R, et al. A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg 2008;107:1026-40. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. Optimal care of patients with non-small cell lung cancer reduces perioperative morbidity. J Thorac Cardiovasc Surg 2011;141:22-33. [Crossref] [PubMed]
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17-41. [Crossref] [PubMed]
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81:1013-9; discussion 1019-20. [Crossref] [PubMed]
- Rostad H, Strand TE, Naalsund A, et al. Lung cancer surgery: the first 60 days. A population-based study. Eur J Cardiothorac Surg 2006;29:824-8. [Crossref] [PubMed]
- Simonsen DF, Søgaard M, Bozi I, et al. Risk factors for postoperative pneumonia after lung cancer surgery and impact of pneumonia on survival. Respir Med 2015;109:1340-6. [Crossref] [PubMed]
- Boffa DJ, Allen MS, Grab JD, et al. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: The surgical management of primary lung tumors. J Thorac Cardiovasc Surg 2008;135:247-54. [Crossref] [PubMed]
- Matsubara Y, Takeda S, Mashimo T. Risk stratification for lung cancer surgery*: Impact of induction therapy and extended resection. Chest 2005;128:3519-25. [Crossref] [PubMed]
- Pastorino U, Borasio P, Francese M, et al. Lung cancer stage is an independent risk factor for surgical mortality. Tumori 2008;94:362-9. [Crossref] [PubMed]
- Spaggiari L, Scanagatta P. Surgery of non-small cell lung cancer in the elderly. Curr Opin Oncol 2007;19:84-91. [Crossref] [PubMed]
- Takamochi K, Oh S, Matsuoka J, et al. Risk factors for morbidity after pulmonary resection for lung cancer in younger and elderly patients. Interact Cardiovasc Thorac Surg 2011;12:739-43. [Crossref] [PubMed]
- Thomas DC, Blasberg JD, Arnold BN, et al. Validating the Thoracic Revised Cardiac Risk Index Following Lung Resection. Ann Thorac Surg 2017;104:389-94. [Crossref] [PubMed]
- Herranz González-Botas J, Varela Vázquez P, Vázquez Barro C. Second primary tumours in head and neck cancer. Acta Otorrinolaringol Esp 2016;67:123-9. [Crossref] [PubMed]
- Vogt A, Schmid S, Heinimann K, et al. Multiple primary tumours: challenges and approaches, a review. ESMO Open 2017;2. [Crossref] [PubMed]
- Salama JK, Stenson KM, List MA, et al. Characteristics associated with swallowing changes after concurrent chemotherapy and radiotherapy in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg 2008;134:1060-5. [Crossref] [PubMed]
- Tschiesner U, Linseisen E, Baumann S, et al. Assessment of functioning in patients with head and neck cancer according to the International Classification of Functioning, Disability, and Health (ICF): a multicenter study. The Laryngoscope 2009;119:915-23. [Crossref] [PubMed]
- Mortensen HR, Jensen K, Grau C. Aspiration pneumonia in patients treated with radiotherapy for head and neck cancer. Acta Oncol 2013;52:270-6. [Crossref] [PubMed]
- Nguyen NP, Smith HJ, Sallah S. Evaluation and management of swallowing dysfunction following chemoradiation for head and neck cancer. Curr Opin Otolaryngol Head Neck Surg 2007;15:130-3. [Crossref] [PubMed]
- Kozower BD, Sheng S, O’Brien SM, et al. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg 2010;90:875-81; discussion 881-3. [Crossref] [PubMed]
- Schussler O, Alifano M, Dermine H, et al. Postoperative pneumonia after major lung resection. Am J Respir Crit Care Med 2006;173:1161-9. [Crossref] [PubMed]
- Wang CY, Lin YS, Tzao C, et al. Comparison of Charlson comorbidity index and Kaplan-Feinstein index in patients with stage I lung cancer after surgical resection. Eur J Cardiothorac Surg 2007;32:877-81. [Crossref] [PubMed]
- Moro-Sibilot D, Aubert A, Diab S, et al. Comorbidities and Charlson score in resected stage I nonsmall cell lung cancer. Eur Respir J 2005;26:480-6. [Crossref] [PubMed]
- Barrera R, Shi W, Amar D, et al. Smoking and timing of cessation: impact on pulmonary complications after thoracotomy. Chest 2005;127:1977-83. [Crossref] [PubMed]
- Rodriguez M, Gómez-Hernandez MT, Novoa N, et al. Refraining from smoking shortly before lobectomy has no influence on the risk of pulmonary complications: a case-control study on a matched population. Eur J Cardiothorac Surg 2017;51:498-503. [PubMed]
- Massard G, Wihlm JM, Ameur S, et al. Association of bronchial and pharyngo-laryngeal malignancies. A reappraisal. Eur J Cardiothorac Surg 1996;10:397-402. [Crossref] [PubMed]
- Herrera LJ, Correa AM, Vaporciyan AA, et al. Increased risk of aspiration and pulmonary complications after lung resection in head and neck cancer patients. Ann Thorac Surg 2006;82:1982-7; discussion 1987-8.
- Xu B, Boero IJ, Hwang L, et al. Aspiration pneumonia after concurrent chemoradiotherapy for head and neck cancer. Cancer 2015;121:1303-11. [Crossref] [PubMed]