Apatinib for EGFR-TKI and chemotherapy refractory in an advanced lung cancer patient: a case report
Introduction
In China, primary lung cancer is one of the most common malignant tumors. The data of China Cancer Center in 2015 showed that the prevalence of lung cancer was 130.2 (1/10 million) from 2006 to 2011 in China. And lung cancer has risen to be the fourth leading cause of death (1). Most patients have distant metastasis when they get initial diagnosis. Despite the rapid development of targeted and chemotherapeutic drugs, there is no standard treatment option after the patients are treated with multiple lines therapy. Apatinib is a new type of small molecule tyrosine kinase inhibitor (TKI) developed by China. It has been approved to selectively bind to vascular endothelial growth factor receptor 2 (VEGFR-2). By inhibiting VEGFR-2, apatinib could decrease VEGF-mediated endothelial cell migration, proliferation, and tumor microvascular density (2-4). A Phase III trial of Apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction showed that median OS was significantly improved in the apatinib group compared with the placebo group (6.5 vs. 4.7 m, P=0.0149), PFS was 2.6 vs. 1.8 m (P<0.001) (5). A phase II clinical trial showed that apatinib compared to the placebo group, the median PFS was significantly prolonged (4.7 vs.1.9 months, P<0.001) after the failure of more than two lines of treatment in NSCLC (6). It is also applied to other solid tumors in clinic work. This case is about one patient treated with apatinib for EGFR-TKI and chemotherapy refractory in an advanced lung cancer patient.
Case presentation
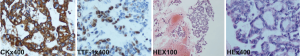
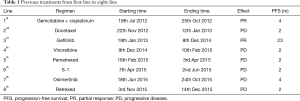
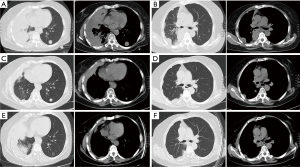
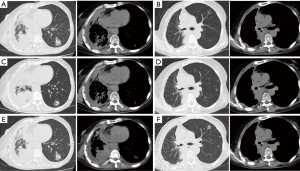
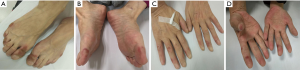
The patient was female, 60 years old, with no other disease and no smoking history. She was admitted for dry cough and chest pain. The chest computed tomography (CT) showed the right lung occupation and pleural metastasis on Jun 2012. Bronchofiberscope was performed and pathological examination revealed adenocarcinoma of the lung (Figure 1). Detection of EGFR gene by tissue showed deletion of exon 19. The patient received several chemotherapy regimens and target therapy for eight lines until 14th Dec 2015 (Table 1). At that time, the patient had an Eastern Cooperative Oncology Group performance status (ECOG) of 2. So she received apatinib at the minimum dose of 250 mg/d. One month later, the chest CT scan showed the intrapulmonary occupation and the pleural metastasis were significant reduction. Symptoms including the cough and chest pain gradually decreased and the score of ECOG was significantly improved. The patient continued apatinib as maintaining therapy and received CT scan every 6 weeks. The effect was maintenance partial response (PR) until 24th Dec 2016 (Figure 2). The ninth-line progression free survival (PFS) was 12 months. But the patient kept taking apatinib until Aug 2017, the lesions were extending gradually (Figure 3). The patient died on Oct 2017, the overall survival (OS) was nearly 5 years. During administration of apatinib, the patient experienced grade 3 hypertension. The blood pressure increased to a maximum of 180/100 mmHg. Valsartan and nifedipine were administered and the blood pressure returned to a normal level. The patient got grade 4 platelet count decreased (15×109/L) after she took apatinib for 90 days. By stopping drug and treating with recombinant human thrombopoietin, the platelet count recovered. The patient also got proteinuria. The urine routine test showed the urinary protein was up to 3+ and the 24 hours total urine protein was 2.8 g. By taking calcium dobesilate capsules and Shenfukang capsules, the urinary protein fluctuated 1+ ~2+ and the 24 hours total urine protein fluctuated 0.6–2.0 g. Other adverse effects included grade 1 oral mucositis and grade 1 palmar-plantar erythrodysesthesia syndrome (Figure 4).
Full table
Discussion
Advanced non-small lung cancer (NSCLC) has poor progress. The median PFS is usually no more than one year. About 30–70% patients can receive more than second-line chemotherapy (3). But there are no standard recommendations for subsequent lines of therapy.
Tumor angiogenesis is a key link in the process of tumor growth and metastasis (7,8). Both vascular endothelial growth factor receptor (VEGFR) and vascular endothelial growth factor (VEGF) play an important role in the process of tumor angiogenesis (9). Bevacizumab combined with chemotherapy has been recommended as a standard treatment for advanced lung adenocarcinoma as first-line therapy. Many clinical trials attempt to evaluate the efficacy of antiangiogenic agent alone on second-line and subsequent lines of therapy in advanced NSCLC. But most of the results did not reach the positive end points (Table 2). In China, apatinib is a new small molecule TKI, it has been approved for the treatment for gastro-esophageal adenocarcinoma or advanced gastric adenocarcinoma as three lines or more than three lines choice. In phase I clinical trials, the maximum tolerated dose of apatinib was 850 mg/day and the recommended dose was 750 mg/day. In this case, the patient was treated with apatinib at 250 mg/d, but she still got 12 months PFS after several lines treatments including EGFR-TKI and chemotherapy. There were no serious adverse reactions to affect the treatment compliance of the patient. The patient received gene test for three times. On 24th Dec 2012, detection of EGFR gene by tissue showed 19 exon mutation. On 24th Sep 2015, after gefitinib and several lines of chemotherapy treatments failed, detection of EGFR gene by blood still showed 19 exon mutation but without T790 resistance gene mutation. On 5th Dec 2016, when the patient had disease progression of apatinib, she received multigene test by bood. The result showed no VEGFR-2 amplification and mutation. It showed EGFR-p.Glu746_Ser752 and EGFR-p.Gly724Ser mutations which were EGFR rare mutation genes and were sensitive to EGFR-TKI (19).

Full table
Another characteristic of the case was the patient took apatinib for another one year after progression. The lesions progressed slowly. It had reported that some patients could benefit from receiving an EGFR-TKI beyond progression (20). But there were no trials of continuing use of antiangiogenic drugs alone after progression. We consider apatinib may affect other pathways to inhibit the tumor progression. Recently, an in vitro study showed that apatinib inhibits cellular invasion and migration in lung cancer cells via suppressing RET/Src signaling pathway (21).
Conclusions
In conclusion, because of the high prices of ramucirumab and nintedanib in China, mono-apatinib might be an option as post second-line treatment in advanced lung adenocarcinoma even at low dose. We believe that with the publication of the results of clinical trials, more evidence will be provided for our clinical application.
Acknowledgements
Funding: This study was supported by National Science and Technology Major Project of the Ministry of Science and Technology of China (No. 2017ZX09304025), Clinical Capability Construction Project for Liaoning Provincial Hospitals (LNCCC-D07-2015), Science and Technology Plan Project of Liaoning Province (No. 2016007010), and the Key Research and Development Program of Shenyang (No. 17-230-9-01).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Zheng R, Zeng HJ, Zhang S. Cancer Lett 2016;370:33-8. [Crossref] [PubMed]
- Li J, Zhao X, Chen L, et al. Safety and pharmacokinetics of novel selective vascularendothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer 2010;10:529. [Crossref] [PubMed]
- Ding J, Chen X, Dai X, et al. Simultaneous determination of apatinib and its four major metabolites in human plasma using liquid chromatography-tandem mass spectrometry and its application to a pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci 2012;895-896:108-15. [Crossref] [PubMed]
- Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 2011;102:1374-80. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from randomized, placebo-controlled, parallel-arm, phase II Trial. J Clin Oncol 2013;31:3219-25. [Crossref] [PubMed]
- Zhang L, Shi M, Huang C, et al. A phase II, multicenter, placebo-controlled trial of apatinib in patients with advanced nonsquamous non-small cell lung cancer (NSCLC) after two previous treatment regimens. J Clin Oncol 2012;30:abstr 7548.
- El-Kenawi AE, El-Remessy AB. Angiogenesis inhibitors in cancer therapy: mechanistic perspective on classification and treatment rationales. Br J Pharmacol 2013;170:712-29. [Crossref] [PubMed]
- Jayson GC, Kerbel R, Ellis LM, et al. Antiangiogenic therapy in oncology: current status and future directions. Lancet 2016;388:518-29. [Crossref] [PubMed]
- Fontanella C, Ongaro E, Bolzonello S, et al. Clinical advances in the development of novel VEGFR2 inhibitors. Ann Transl Med 2014;2:123. [PubMed]
- Weiss JM, Villaruz LC, Socinski MA, et al. A single-arm phase II trial of pazopanib in patients with advanced non-small cell lung cancer with non-squamous histology with disease progression on bevacizumab containing therapy. Lung Cancer 2014;86:288-90. [Crossref] [PubMed]
- Leighl NB, Raez LE, Besse B, et al. A multicenter, phase 2 study of vascular endothelial growth factor trap (Aflibercept) in platinum- and erlotinib-resistant adenocarcinoma of the lung. J Thorac Oncol 2010;5:1054-9. [Crossref] [PubMed]
- Lee JS, Hirsh V, Park K, et al. Vandetanib Versus placebo in patients with advanced non-small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double-blind phase III trial (ZEPHYR). J Clin Oncol 2012;30:1114-21. [Crossref] [PubMed]
- Natale RB, Thongprasert S, Greco FA, et al. Phase III trial of vandetanib compared with erlotinib in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 2011;29:1059-66. [Crossref] [PubMed]
- Socinski MA, Novello S, Brahmer JR, et al. Multicenter, phase II trial of sunitinib in previously treated, advanced non-small-cell lung cancer. J Clin Oncol 2008;26:650-6. [Crossref] [PubMed]
- Tan EH, Goss GD, Salgia R, et al. Phase 2 trial of Linifanib (ABT-869) in patients with advanced non-small cell lung cancer. J Thorac Oncol 2011;6:1418-25. [Crossref] [PubMed]
- Paz-Ares L, Hirsh V, Zhang L, et al. Monotherapy Administration of Sorafenib in Patients With Non-Small Cell Lung Cancer (MISSION) Trial: A Phase III, Multicenter, Placebo-Controlled Trial of Sorafenib in Patients with Relapsed or Refractory Predominantly Nonsquamous Non-Small-Cell Lung Cancer after 2 or 3 Previous Treatment Regimens. J Thorac Oncol 2015;10:1745-53. [Crossref] [PubMed]
- Zhou Q, Zhou CC, Chen GY, et al. A multicenter phase II study of sorafenib monotherapy in clinically selected patients with advanced lung adenocarcinoma after failure of EGFR-TKI therapy (Chinese Thoracic Oncology Group, CTONG 0805). Lung Cancer 2014;83:369-73. [Crossref] [PubMed]
- Blumenschein GR Jr, Gatzemeier U, Fossella F, et al. Phase II, multicenter, uncontrolled trial of single-agent sorafenib in patients with relapsed or refractory, advanced non-small-cell lung cancer. J Clin Oncol 2009;27:4274-80. [Crossref] [PubMed]
- Zhu X, Bai Q, Lu Y, et al. Responce to Tyrosine Kinase Inhibitors in Lung Adenocarcinoma with the Rare Epidermal Growth Factor Receptor Mutation S768I:a Retrospective Analysis and Literature Review. Target Oncol 2017;12:81-8. [Crossref] [PubMed]
- Goto Y, Tanai C, Yoh K, et al. Continuing EGFR-TKI beyond radiological progression in patients with advanced or recurrent, EGFR mutation-positive non-small-cell lung cancer: an observational study. ESMO Open 2017;2. [Crossref] [PubMed]
- Lin C, Wang S, Xie W, et al. Apatinib inhibits cellular invasion and migration by fusion kinase KIF5B-RET via suppressing RET/Src signaling pathway. Oncotarget 2016;7:59236-44. [PubMed]


