Atezolizumab in non-squamous non-small cell lung cancer
Immune therapy opened a new era in treatment of advanced non-small cell lung cancer (NSCLC). Immune checkpoint inhibitors (ICIs) targeting the programmed death-ligand 1 (PD-L1)/programmed death-1 (PD-1) pathway have achieved great success over conventional cytotoxic chemotherapy. Several anti-PD-1 antibodies, including nivolumab and pembrolizumab, were approved by the Food and Drug Administration (FDA) in 2015 for use as second-line therapy for advanced NSCLC. The drugs were approved after demonstrating both clinical efficacy and an improved safety profile compared with standard docetaxel for the treatment of non-squamous NSCLC (1,2). Pembrolizumab received FDA approval in 2016 as a first-line treatment for patients with advanced NSCLC and high PD-L1 expressing tumors (3,4).
The PD-L1 antibody, atezolizumab (Tecentriq, F. Hoffmann-La Roche/Genentech), is a selective humanized monoclonal IgG1 antibody, which was designed to block the interaction between PD-L1 and the PD-1/B7-1 activation complex, but not bind to PD-L2 (5). This antibody showed efficacy in the open-label randomized phase III OAK trial (NCT02008227) which led to its approval as a second-line therapy for advanced NSCLC (6). Efficacy was noted in both squamous and non-squamous NSCLC subsets and regardless of PD-L1 status (6). However, efficacy of ICIs in patients with NSCLC harboring epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) rearrangement was controversial in previous trials (1,2,6).
Following the OAK trial, the phase III IMpower150 study evaluating atezolizumab for first-line treatment was initiated and the results were published in the New England Journal Medicine (7). The aim of this trial was to compare the efficacy and safety of atezolizumab in combination with carboplatin and paclitaxel with or without bevacizumab in patients with stage IV non-squamous NSCLC who had not previously received chemotherapy. A total of 1,202 patients were enrolled and randomized (1:1:1) to receive: atezolizumab plus carboplatin and paclitaxel (ACP group); atezolizumab and bevacizumab plus carboplatin and paclitaxel (ABCP group); bevacizumab plus carboplatin and paclitaxel (BCP group). The entire intention-to-treat population (ITT) was divided into two groups: patients with a wild-type genotype (ITT-WT; patients without EGFR or ALK alterations); or patients with EGFR or ALK alterations (ITT-EGFR/ALK+). Within the ITT-WT group, patients with high expression of an effector T-cell (Teff) gene signature in the tumor were categorized as Teff-high WT population. PD-L1 expression was evaluated by SP142 antibody.
The two primary end points were progression-free survival (PFS) in ITT-WT and Teff-high WT populations, and overall survival (OS) in the ITT-WT population. In addition, the following key secondary end points were assessed: PFS and OS in the ITT-EGFR/ALK+ group; PFS in the PD-L1 expression subgroups; toxicity in the ITT group.
In the ITT-WT population, median PFS was significantly longer in the ABCP group compared with the BCP group [8.3 vs. 6.8 months, respectively; hazard ratio (HR), 0.62; 95% confidence interval (CI), 0.52–0.74; P=0.0003]. The results of subgroup analysis within the ITT group were as follow: PFS for ITT-EGFR/ALK+ patients were longer with ABCP treatment than with BCP (9.7 vs. 6.1 months, respectively; unstratified HR, 0.59; 95% CI, 0.37–0.94); PFS in patients with low or negative PD-L1 expression was longer with ABCP treatment than with BCP (8.0 vs. 6.6 months, respectively; unstratified HR, 0.68; 95% CI, 0.56–0.82], this trend was also observed for the subgroup of patients with high PD-L1 expression. The safety profile of ABCP treatment was consistent with those of the other groups as well as previous reports of each individual medication (6).
The most important finding of the trial was that ICI in combination with chemotherapy significantly prolonged PFS and OS in the WT group compared with conventional therapy alone. This IMpower150 study is the second phase III trial investigating the additional effect of ICIs to conventional therapy, the first was the KEYNOTE-189 trial (pembrolizumab + platinum/pemetrexed doublet) (8). In 2017, using the results of the KEYNOTE-021 phase II study, the FDA approved pembrolizumab combined with a pemetrexed and carboplatin regimen as first-line treatment for non-squamous NSCLC, regardless of PD-L1 expression. However, there was no benefit to OS between the two groups (9). A recent meta-analysis of data from trials using this type of combination therapy showed that there was a significant improvement in PFS but not OS (10). Overall, controversy remains regarding whether such combination therapy for first-line treatment is able to prolong OS. There are numerous ongoing phase III trials investigating the effects of similar combination therapies. For instance, the IMpower130 (atezolizumab + platinum-based chemotherapy + pemetrexed) and 132 (atezolizumab + platinum-based chemotherapy + nab-paclitaxel) trials are currently ongoing (Figure 1) (11). We eagerly await the results of these studies in hopes they will clear up the current controversy surrounding the efficacy of first-line combination therapy.
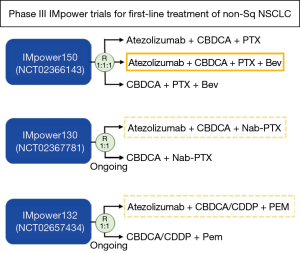
The IMpower150 trial was the first published trial investigating the efficacy of combination therapy with ICI and anti-angiogenetic agents. Anti-angiogenic agents targeting vascular endothelial growth factor (VEGF) or VEGF receptor include bevacizumab, ramucirumab and nintedanib. Currently, four ongoing trials are investigating the effects of combination therapy with anti-angiogenetic agents in NSCLC patients, the trials are described as follows: NCT01454102 (nivolumab + bevacizumab), NCT02039674 (pembrolizumab + bevacizumab), NCT02443324 (pembrolizumab + ramucirumab), NCT02856425 (pembrolizumab + nintedanib) (12). Over the past decades, it has become apparent that VEGF, known as the principal regulator of angiogenesis, also has an immunosuppressive role which enables tumor cells to evade immunosurveillance (13). The immunosuppressive functions of VEGF are summarized as follows: influence on induction and expansion of inhibitory immune cell subsets, such as T-regulatory and myeloid-derived suppressor cells; suppression of dendritic cell maturation; mitigation of effector T-cell response; alteration of lymphocyte development and trafficking (12,14) (Figure 2). Preclinical experiments have demonstrated the potential efficacy of combining ICIs with anti-angiogenetic agents (15). Therefore, it is expected that the addition of anti-angiogenetic agents will further improve the outcome for patients treated with ICIs (16).
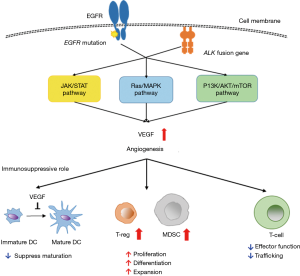
An interesting result of the IMpower150 trial was that the benefit of ABCP treatment was observed in patients with EGFR or ALK genetic alterations, although it was an exploratory subgroup analysis. One speculation for the benefit of ABCP combination therapy is that it was a result of the additional effect of bevacizumab, an anti-VEGF monoclonal antibody. Biologically, the EGFR pathway regulates VEGF expression via multiple pathways (Figure 2), leading us to hypothesize that VEGF signal inhibition is effective for tumors harboring EGFR mutation (17). In fact, there are several trials investigating the additional effect of bevacizumab in patients with advanced NSCLC harboring EGFR mutations that are being treated with EGFR-tyrosine kinase inhibitor (TKI) (18,19). In a phase II study (JO25567), Seto et al. demonstrated that treatment with a combination of bevacizumab + erlotinib significantly prolonged PFS compared with erlotinib alone (18). Furthermore, preclinical studies have also shown that anti-VEGF was effective against tumors harboring EGFR-TKI resistance mutations (20). Although further clinical studies are warranted, these early studies indicate that combination therapy with ICI and anti-angiogenetic agents could be another option for treatment in patients with EGFR or ALK genetic alterations for which TKI therapy has failed. This new therapy option may prolong survival in these patients.
The safety profile of ABCP treatment was consistent with those of the other groups as well as those reported for each individual medication. The incidence rate of immune-related adverse events (irAEs) in the ABCP group was similar to that observed with atezolizumab monotherapy in the OAK III trial (6). However, the incidence rate of pneumonitis, an irAE, was higher in the ABCP group than in the BCP group: all grade, 2.8% vs. 1.3%, respectively; grade 3–4, 1.5% vs. 0.5%, respectively. In the OAK III trial, the incidence rate of pneumonitis was 1% in all grade and less than 1% in grade 3–4. In addition, recent meta-analysis of pneumonitis incidence with use of PD-L1 inhibitors demonstrated that the incidence rate was 1.3% (95% CI, 0.8–1.9%) in all grade pneumonitis and 0.4% (95% CI, 0–0.8%) in grade 3–4 pneumonitis (21). Therefore, the incident rate of pneumonitis in the ABCP group was somewhat higher than previously reported. The potential risk of pneumonitis while treating with ICIs should be noted.
An important piece of data analysis that the authors would like to see from the IMpower150 trial is the additional effect of bevacizumab to patients treated with ICIs, which was not mentioned in the paper. This trial was designed as a three-arm study, therefore, it is unclear why the analysis of comparison between the ABCP and ACP treatment groups was excluded from the outcome measure. Such subgroup analysis should be performed to elucidate the additional effect of anti-angiogenetic agents when combined with ICIs.
Another expectation of combination therapy with ICIs and anti-angiogenetic agents is the benefit of targeting central nervous system (CNS) metastasis. Patients with active or untreated CNS metastases were excluded from the IMpower150 trial. The efficacy of ICIs to treat CNS metastases has been explored and at present, a consensus has not been reached (22); however, subgroup analysis of the OAK phase III trial demonstrated that atezolizumab improved the OS in patients with CNS metastases (6). Bevacizumab combined with chemotherapy demonstrated promising activity against CNS metastases in the BRAIN trial and current guidelines permit to its use when CNS metastases are present (23). If ABCP therapy is approved, it will be useful to understand the effect of ABCP treatment on CNS metastases.
The antibody used for PD-L1 immunohistochemistry (IHC) assays as well as the scoring system for determining PD-L1 expression must be discussed. The scoring system used in the IMpower150 was different from that in other trials: PD-L1 expression was evaluated on tumor cells as well as on the surrounding tumor-infiltrating immune cells, but other trials evaluated PD-L1 expression only on tumor cells. In addition, clinical trials for atezolizumab used the SP142 antibody for the PD-L1 IHC assay, but the clinical trial for pembrolizumab used the 22C3 antibody. Because of the potential inconsistency of results when using different scoring systems and IHC antibodies, it is important that future clinical trials testing the efficacy of ICIs alone or in combination with other medication, conduct IHC assays using the same PD-L1 antibody and scoring system to reduce confusion and allow for results to be confidently compared among trials.
The significance of treating with a combination of ICI and conventional therapies, including bevacizumab, was successfully demonstrated in the IMpower150 trial. Though the above-mentioned issues have yet to be clarified, combination therapy of atezolizumab with bevacizumab + chemotherapy may become an optional standard-of-care for first-line treatment of non-squamous NSCLC. Options for first-line treatment of non-squamous NSCLC will be discussed further in the near future.
Acknowledgements
We thank Sarah Bubeck, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Footnote
Conflicts of Interest: Takashi Seto reports grants from Bayer Yakuhin, Eisai, Merck Serono, Novartis Pharma, and Verastem; personal fees from Bristol-Myers Squibb, Kyowa Hakko Kirin, Mochida Pharmaceutical, Nippon Kayaku, Ono Pharmaceutical, Roche Singapore, Sanofi, Showa Yakuhin, Taiho Pharmaceutical and Takeda Pharmaceutical; grants and personal fees from Astellas Pharma, AstraZeneca, Chugai Pharmaceutical, Daiichi Sankyo, Eli Lilly Japan, Kissei Pharmaceutical, MSD, Nippon Boehringer Ingelheim, Pfizer Japan, YakultHonsha, outside the submitted work. The other authors have no conflicts of interest to declare.
References
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Pai-Scherf L, Blumenthal GM, Li H, et al. FDA Approval Summary: Pembrolizumab for Treatment of Metastatic Non-Small Cell Lung Cancer: First-Line Therapy and Beyond. Oncologist 2017;22:1392-9. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Stewart R, Morrow M, Hammond SA, et al. Identification and Characterization of MEDI4736, an Antagonistic Anti-PD-L1 Monoclonal Antibody. Cancer Immunol Res 2015;3:1052-62. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Papadimitrakopoulou V, Gadgeel SM, Borghaei H, et al. First-line carboplatin and pemetrexed (CP) with or without pembrolizumab (pembro) for advanced nonsquamous NSCLC: Updated results of KEYNOTE-021 cohort G. J Clin Oncol 2017;35:abstr 9094.
- Xu X, Huang Z, Zheng L, et al. The efficacy and safety of anti-PD-1/PD-L1 antibodies combined with chemotherapy or CTLA4 antibody as a first-line treatment for advanced lung cancer. Int J Cancer 2018;142:2344-54. [Crossref] [PubMed]
- Papadimitrakopoulou V, Cappuzzo F, Jotte RM, et al. Phase III clinical trials of atezolizumab combined with chemotherapy in chemotherapy-naive patients with advanced NSCLC. J Clin Oncol 2016;34:abstr TPS9103.
- Manegold C, Dingemans AC, Gray JE, et al. The Potential of Combined Immunotherapy and Antiangiogenesis for the Synergistic Treatment of Advanced NSCLC. J Thorac Oncol 2017;12:194-207. [Crossref] [PubMed]
- Ohm JE, Carbone DP. VEGF as a mediator of tumor-associated immunodeficiency. Immunol Res 2001;23:263-72. [Crossref] [PubMed]
- Voron T, Marcheteau E, Pernot S, et al. Control of the immune response by pro-angiogenic factors. Front Oncol 2014;4:70. [Crossref] [PubMed]
- Li B, Lalani AS, Harding TC, et al. Vascular endothelial growth factor blockade reduces intratumoral regulatory T cells and enhances the efficacy of a GM-CSF-secreting cancer immunotherapy. Clin Cancer Res 2006;12:6808-16. [Crossref] [PubMed]
- Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol 2018;15:310-24. [Crossref] [PubMed]
- Naumov GN, Nilsson MB, Cascone T, et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res 2009;15:3484-94. [Crossref] [PubMed]
- Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 2014;15:1236-44. [Crossref] [PubMed]
- Rosell R, Dafni U, Felip E, et al. Erlotinib and bevacizumab in patients with advanced non-small-cell lung cancer and activating EGFR mutations (BELIEF): an international, multicentre, single-arm, phase 2 trial. Lancet Respir Med 2017;5:435-44. [Crossref] [PubMed]
- Larsen AK, Ouaret D, El Ouadrani K, et al. Targeting EGFR and VEGF(R) pathway cross-talk in tumor survival and angiogenesis. Pharmacol Ther 2011;131:80-90. [Crossref] [PubMed]
- Khunger M, Rakshit S, Pasupuleti V, et al. Incidence of Pneumonitis With Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Trials. Chest 2017;152:271-81. [Crossref] [PubMed]
- Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016;17:976-83. [Crossref] [PubMed]
- Besse B, Le Moulec S, Mazieres J, et al. Bevacizumab in Patients with Nonsquamous Non-Small Cell Lung Cancer and Asymptomatic, Untreated Brain Metastases (BRAIN): A Nonrandomized, Phase II Study. Clin Cancer Res 2015;21:1896-903. [Crossref] [PubMed]


