Elevated interleukin-6 and bronchiectasis as risk factors for acute exacerbation in patients with tuberculosis-destroyed lung with airflow limitation
Introduction
Tuberculosis-destroyed lung (TDL) is a condition in which pulmonary tuberculosis (TB) has caused destructive changes in the pulmonary parenchyma over the years, leading to chronic respiratory obstruction (1). Patients with TDL have clinical characteristics similar to those of patients with chronic obstructive pulmonary disease (COPD), such as dyspnea, sputum production, and acute exacerbation (2). Acute exacerbations appear to accelerate the decline in lung function (3), resulting in a poorer quality of life (4) and an increased risk of death in patients with COPD (5). Exacerbations might be important outcomes in TDL, as they are in COPD, and their prevention is a key component of TDL (6). Airflow limitation is an independent risk factor for acute exacerbation in TDL patients (7). However, not many previous studies have evaluated the risk factors of acute exacerbation in patients with TDL with airflow limitation.
Recent studies have suggested that TDL is a dynamic systemic inflammatory disease (1). Patients with COPD combined with TDL or bronchiectasis are known to have worse outcomes because of recurrent airway infection and systemic inflammation, which are usually quantified by serum inflammatory markers [C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and interleukin (IL)-6] (2,8,9). Thus, these inflammatory markers might also be associated with acute exacerbation in TDL with airflow limitation (2,8,9).
Treatment with a bronchodilator improves the clinical outcomes of TDL (10,11). Additionally, management and prevention of inflammation, especially in patients with the risk of acute exacerbation, is important in TDL with airflow limitation. The aim of this study was to investigate the factors associated with acute exacerbation in patients with TDL with airflow limitation.
Methods
Study design
In this multicenter prospective cohort study, we enrolled a cohort of patients with chronic airway disease who presented to Korea University Guro Hospital, Seoul St. Mary’s Hospital, and Chonbuk National University Hospital. This study was conducted to develop optimal clinical and biological markers to facilitate diagnosis and monitoring of treatment responses in patients with chronic airway disease from May 2015. The inclusion criteria for the chronic airway disease group were as follows: adult patients, aged over 19 years, who were clinically diagnosed with asthma and chronic obstructive lung disease. Among these, we selected patients with chronic airflow limitation due to TDL, in stable status, without exacerbation in the 3 months prior to consultation at the hospitals mentioned above, between June 2015 and May 2016. This study protocol was approved by the relevant institutional review boards (Korea University Guro Hospital: KUGH 13246, Seoul St. Mary’s Hospital: KC15OIMI0553, Chonbuk National University Hospital: 2015-01-018-005). Written informed consent was obtained from each patient, and the study conformed to the tenets of the Declaration of Helsinki.
Study subjects
A diagnosis of TDL with chronic airflow limitation was made based on the following criteria: (I) a history of TB and no change in chest imaging test over the past 1 year; (II) at least one finding of destroyed pulmonary parenchyma on a chest image (lung volume loss, bronchovascular distortion, fibrosis, or bronchiectasis) and the sum of the volume of all lesions equivalent to 25% of one lung, as confirmed by a radiologist or pulmonologist; (III) airflow limitation [post-bronchodilator spirometry with forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) <70%], and no history of asthma or COPD before the diagnosis of TB; (IV) respiratory infection within the previous 3 months.
Clinical parameters
We evaluated the following clinical parameters at baseline: smoking history (pack-years and smoking status), past medical history, past exacerbation history, pulmonary function test [spirometry, lung volume, diffusing capacity of the lung for carbon monoxide (DLCO)], plain chest radiography, chest computed tomography (CT), FEV1 decline rates, number of exacerbations per year, St. George Respiratory Questionnaire (SGRQ) results, COPD assessment test score, and blood sample measurements.
Pulmonary function test reference values were calculated using the equation proposed by Choi et al. (12). Follow-up pulmonary function tests were performed at 1 year after enrolment, regardless of the presence of exacerbation. The FEV1 decline rate was calculated using the change in FEV1 at 1 year after enrollment, at stable status. Exacerbation was defined as an unscheduled visit during which the patient required emergency care with antibiotics or systemic steroid because of worsened respiratory symptoms (cough, sputum, or dyspnea).
Radiologic findings
The extent of destruction was graded into three categories. If the extent of destruction on chest radiograph was less than one-third of the hemithorax, it was classified as grade 1. If the extent of destruction was between one-third and two-thirds, it was classified as grade 2. If the extent of destruction was more than two-thirds, it was classified as grade 3. Destroyed lung patterns on CT findings, including the presence of bronchiectasis, pleural thickening, constrictive bronchiolitis, bronchostenosis, and emphysema, were evaluated by one radiologist and one pulmonology specialist. Bronchiectasis was defined as irreversible dilatation of the bronchi, resulting in dilated, thick-walled airways typically extending toward the lung periphery (13). The severity of bronchiectasis was evaluated using the FACED score (14,15), which comprises FEV1, age, Pseudomonas aeruginosa colonization, radiological extension, and dyspnea (mild: 0–2 points; moderate: 3–4 points; severe: 5–7 points). Constrictive bronchiolitis was found in CT as thickening of the wall of the bronchioles, which might directly lead to a centrilobular branching structure, or indirectly via mosaic perfusion or air trapping (16). Emphysema was defined as an abnormal permanent enlargement of the airspaces distal to the terminal bronchioles, accompanied by destruction of the alveolar wall, without obvious fibrosis.
Serum laboratory findings
Blood tests, including complete blood cell count with differentiation, and measurement of CRP level and ESR, were performed routinely on the day of enrollment, as baseline values, when the patient was in a stable state. Peripheral whole-venous blood was collected into ethylenediamine tetra-acetic acid tubes, and serum was prepared by centrifugation for 10–15 min at 4,500 rpm and stored at −80 °C until analyzed. IL-6 was measured using enzyme-linked immunosorbent assay kits (IL-6: R&D Systems, Oxford, UK).
Statistical analysis
Clinical data were presented as the median and interquartile range for continuous variables and as a number (percentage) for categorical variables. Data were compared using the Mann-Whitney U-test for continuous variables and Pearson’s χ2 test or Fisher’s exact test for categorical variables. Correlations between the plasma biomarkers and parameters were analyzed using Pearson’s or Spearman’s correlation analysis. Statistical significance was defined as P<0.05. All statistical analyses were performed using IBM SPSS Statistics for Windows Version 20.0 (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics
A total of 92 patients were enrolled. Their baseline characteristics are described in Table 1. The median age of patients was 67.0 (63.0–71.8) years and 66.3% were men. The median smoking amount was 0.0 (0.0–30.0) pack-year and FEV1 was 1.60 (1.18–2.00) L, and 57.5%pred (45.0–72.5%pred). No patients had started home oxygen therapy or noninvasive positive-pressure ventilation.
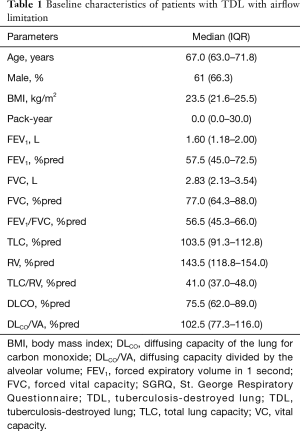
Full table
Baseline characteristics according to exacerbation
Patients with exacerbations had a significantly higher CAT score [patients with exacerbation, 20.0 (14.3–28.0); patients without exacerbation 14.5 (9.8–19.3), P=0.009] and SGRQ score [patients with exacerbation, 26.3 (16.0–46.9); patients without exacerbation 19.9 (13.2–31.8), P=0.047], and tended to have a lower trend for FEV1 (Table 2). Patients with exacerbation within the previous 1 year were more likely to experience acute exacerbations. Routine sputum studies were not performed and previous sputum study results were available in only 32 patients. Pseudomonas aeruginosa was identified in 14 of these patients. Among 23 patients with colonization, 11 experienced acute exacerbation and 12 did not experience acute exacerbation.

Full table
Relationship of radiologic findings and exacerbation
When we investigated the CT findings of patients with TDL, 43.5% were found to have bronchiectasis, 39.1% had pleural thickening, 26.4% had constrictive bronchiolitis, 54.9% had emphysema, and 8.8% had bronchostenosis. Patients with TDL and bronchiectasis and emphysema had more exacerbations (bronchiectasis, r=0.356, P=0.001; emphysema, r=0.233, P=0.026). The presence of colonization was positively associated with the presence of bronchiectasis (Spearman’s correlation: r=0.304, P=0.003). Pleural thickening, bronchostenosis, and the extent of destruction were not associated with serum inflammatory markers or outcomes.
Incidence of bronchiectasis was higher in patients with exacerbation than in those without exacerbation (patients with exacerbation, 66.7%; patients without exacerbation, 30.5%; P=0.001; Table 3). Other CT findings, including the extent of destruction, did not achieve statistical significance.

Full table
Relationship between serum laboratory findings and exacerbation
IL-6 was positively correlated with the FEV1 decline rate (r=0.324, P=0.002) and the number of exacerbations per year (r=0.412, P<0.001) in patients with TDL. CRP and IL-6 levels were significantly higher in patients with exacerbation than in those without exacerbation (P=0.001 and P<0.001, respectively; Table 4).

Full table
Risk factors associated with exacerbation
Using univariate logistic regression, CAT (OR 1.081, 95% CI, 1.020–1.147, P=0.009), previous exacerbation (OR 3.611, 95% CI, 1.370–9.516, P=0.009), bronchiectasis (OR 4.556, 95% CI, 1.831–11.335, P=0.001]), CRP (OR 1.110, 95% CI, 1.000–1.231, P=0.049), and IL-6 (OR 1.148, 95% CI, 1.047–1.259, P=0.003) were associated with acute exacerbation in patients with TDL with airflow limitation. Variables with a P value <0.1 on univariate analysis were analyzed using multivariate logistic regression analysis. Multivariate logistic regression was performed with adjustment for FEV1%pred, FEV1 decline rate per year, CAT, previous exacerbation, SGRQ, bronchiectasis, CRP, and IL-6. Bronchiectasis (OR 3.248, 95% CI, 1.063–9.928, P=0.039) and IL-6 (OR 1.128, 95% CI, 1.013–1.257, P=0.028) were the most important factors associated with acute exacerbation (Table 5). After adjustment, the severity of bronchiectasis was also a significant predictor of acute exacerbation (adjusted OR 2.050, 95% CI, 1.100–3.822, P=0.024).
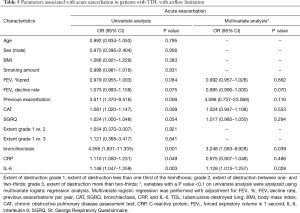
Full table
Discussion
This study revealed that patients with a poor quality of life and symptom scores experienced more frequent exacerbations. The presence of bronchiectasis and high IL-6 levels could be risk factors of future exacerbations in patients with TDL with airflow limitation.
TDL has recently been considered more likely to be a progressive inflammatory disease with lung function decline, similar to COPD (1). A recent study demonstrated that the levels of inflammatory cytokines remained high (17,18), and that lung remodeling and chronic inflammatory responses may persist after TB has been cured (6). Moreover, coexistent bronchiectasis and bacterial colonization in TDL may cause repetitive infection and inflammation which frequently lead to exacerbation (8,9,19). When we evaluated CT findings in our study, patients with bronchiectasis experienced more exacerbations. Moreover, bronchiectasis severity was also associated with acute exacerbation. As colonization was positively associated with the presence of bronchiectasis, we assumed that patients with TDL and bronchiectasis might have pathogenic infections that could lead to repetitive infection and chronic inflammation of the airways. These results imply that a lung destroyed by TB and repetitive infection may contribute to systemic inflammatory responses. Our study showed that CRP and IL-6 were related to lung function decline and exacerbation, as in COPD (20-22). Therefore, as in COPD, the treatment and control of the inflammatory response should be a focus in patients with TDL with airflow limitation.
Patients with TDL have severe symptoms and exacerbation, with a significant burden of morbidity and mortality (1,2) but there is currently no standard treatment for such patients. Recent studies have shown that long-term bronchodilator use in patients with TDL appears to delay the disease course (23), but the role of other medications that modulate systemic inflammation has not been well investigated in TDL. In this study, baseline symptom scores and inflammatory biomarkers were higher and quality of life was worse in patients with exacerbation. These results suggest the need for an additional treatment strategy for modulating systemic inflammation in TDL in order to prevent exacerbation.
Prediction of exacerbation, which is a key factor in disease progression, is important for controlling chronic inflammatory airway disease (24-26). To our knowledge, no previous study has investigated the serum inflammatory markers for predicting exacerbation in TDL. We suggest that IL-6 may be a good marker for predicting the prognosis of TDL. Regarding bronchiectasis, COPD-related bronchiectasis is associated with increased respiratory infection, exacerbation, and mortality (8,9,27). We confirmed that bronchiectasis, rather than decreased lung function or the extent of destruction, is a risk factor in patients with TDL with airflow limitation. Additional studies with larger sample sizes are needed to confirm this finding.
Our study had some limitations. First, the characteristics and biomarkers of TDL without airflow obstruction were not evaluated. However, previous reports suggested that most TDL patients had airflow obstruction (1,28) and that patients with TDL with airflow limitation are more likely to experience exacerbation (2). Thus, we selected patients with airflow limitation. Second, as sputum studies were not performed in all patients, there could be a bias for including sputum culture results as a risk factor of acute exacerbation. Thus, we excluded the sputum culture results in the final evaluation of risk factors for acute exacerbation. Third, the IL-6 levels of a normal control population were not compared with those of the patients enrolled in our study. However, when we compared the IL-6 levels of COPD patients in our cohort (n=78), IL-6 levels were higher in patients with TDL than in patients with COPD [TDL, 2.17 (1.11–5.86) pg/mL; COPD, 1.44 (0.43–2.35) pg/mL; P<0.001]. The IL-6 levels of patients with COPD are known to be higher than those of normal controls (29). This implies that patients with TDL with airflow limitation might have higher IL-6 levels than do normal controls.
Despite these limitations, this study revealed factors associated with acute exacerbation in patients with TDL with airflow limitation. Further larger studies are warranted to confirm our findings.
Conclusions
TDL patients who have bronchiectasis on CT and high serum IL-6 levels might require more intensive control of inflammation to prevent exacerbation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study protocol was approved by our institutional review board (Korea University Guro Hospital: KUGH 13246, Seoul St. Mary’s Hospital: KC15OIMI0553, Chonbuk National University Hospital: 2015-01-018-005). Written informed consent was obtained from each of the patients and the study conformed to the tenets of the Declaration of Helsinki.
References
- Rhee CK, Yoo KH, Lee JH, et al. Clinical characteristics of patients with tuberculosis-destroyed lung. Int J Tuberc Lung Dis 2013;17:67-75. [Crossref] [PubMed]
- Ryu YJ, Lee JH, Chun EM, et al. Clinical outcomes and prognostic factors in patients with tuberculous destroyed lung. Int J Tuberc Lung Dis 2011;15:246-50. [PubMed]
- Donaldson GC, Seemungal T, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57:847-52. [Crossref] [PubMed]
- Miravitlles M, Ferrer M, Pont A, et al. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax 2004;59:387-95. [Crossref] [PubMed]
- Soler-Cataluña JJ, Martínez-García MÁ, Sanchez PR, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005;60:925-31. [Crossref] [PubMed]
- Byrne AL, Marais BJ, Mitnick CD, et al. Tuberculosis and chronic respiratory disease: a systematic review. Int J Infect Dis 2015;32:138-46. [Crossref] [PubMed]
- Kim SJ, Lee J, Park YS, et al. Effect of airflow limitation on acute exacerbations in patients with destroyed lungs by tuberculosis. J Korean Med Sci 2015;30:737-42. [Crossref] [PubMed]
- Gatheral T, Kumar N, Sansom B, et al. COPD-related bronchiectasis; independent impact on disease course and outcomes. COPD 2014;11:605-14. [Crossref] [PubMed]
- Mao B, Lu HW, Li MH, et al. The existence of bronchiectasis predicts worse prognosis in patients with COPD. Sci Rep 2015;5:10961. [Crossref] [PubMed]
- Yum HK, Park IN. Effect of inhaled tiotropium on spirometric parameters in patients with tuberculous destroyed lung. Tuberc Respir Dis (Seoul) 2014;77:167-71. [Crossref] [PubMed]
- Kim CJ, Yoon HK, Park MJ, et al. Inhaled indacaterol for the treatment of COPD patients with destroyed lung by tuberculosis and moderate-to-severe airflow limitation: results from the randomized InFInITY study. Int J Chron Obstruct Pulmon Dis 2017;12:1589. [Crossref] [PubMed]
- Choi JK, Paek D, Lee JO. Normal predictive values of spirometry in Korean population. Tuberc Respir Dis 2005;58:230-42. [Crossref]
- McGuinness G, Naidich D, Leitman B, et al. Bronchiectasis: CT evaluation. AJR Am J Roentgenol 1993;160:253-9. [Crossref] [PubMed]
- Martínez-García MÁ, de Gracia J, Relat MV, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis. The FACED score. Eur Respir J 2014;43:1357-67. [Crossref] [PubMed]
- Guan WJ, Chen RC, Zhong NS. The bronchiectasis severity index and FACED score for bronchiectasis. Eur Respir J 2016;47:382-4. [Crossref] [PubMed]
- Müller NL, Miller RR. Diseases of the bronchioles: CT and histopathologic findings. Radiology 1995;196:3-12. [Crossref] [PubMed]
- Ugarte-Gil CA, Elkington P, Gilman RH, et al. Induced sputum MMP-1, -3 & -8 concentrations during treatment of tuberculosis. PLoS One 2013;8. [Crossref] [PubMed]
- Radovic M, Ristic L, Ciric Z, et al. Changes in respiratory function impairment following the treatment of severe pulmonary tuberculosis-limitations for the underlying COPD detection. Int J Chron Obstruct Pulmon Dis 2016;11:1307. [Crossref] [PubMed]
- Jordan TS, Spencer EM, Davies P. Tuberculosis, bronchiectasis and chronic airflow obstruction. Respirology 2010;15:623-8. [Crossref] [PubMed]
- Higashimoto Y, Iwata T, Okada M, et al. Serum biomarkers as predictors of lung function decline in chronic obstructive pulmonary disease. Respir Med 2009;103:1231-8. [Crossref] [PubMed]
- Hurst JR, Donaldson GC, Perera WR, et al. Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;174:867-74. [Crossref] [PubMed]
- Ferrari R, Tanni SE, Caram LM, et al. Three-year follow-up of interleukin 6 and C-reactive protein in chronic obstructive pulmonary disease. Respir Res 2013;14:24. [Crossref] [PubMed]
- Eom JS, Park HY, Lee MK, et al. The effects of bronchodilators in patients with tuberculosis-destroyed lung and chronic airflow obstruction. Eur Respir J 2015;46.
- Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest 2003;124:459-67. [Crossref] [PubMed]
- Connors AF Jr, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med 1996;154:959-67. [Crossref] [PubMed]
- Wedzicha JA, Donaldson GC. Exacerbations of chronic obstructive pulmonary disease. Respir Care 2003;48:1204-13; discussion 1213-5. [PubMed]
- Patel IS, Vlahos I, Wilkinson TM, et al. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;170:400-7. [Crossref] [PubMed]
- Willcox PA, Ferguson A. Chronic obstructive airways disease following treated pulmonary tuberculosis. Respir Med 1989;83:195-8. [Crossref] [PubMed]
- Wei J, Xiong XF, Lin YH, et al. Association between serum interleukin-6 concentrations and chronic obstructive pulmonary disease: a systematic review and meta-analysis. PeerJ 2015;3. [Crossref] [PubMed]


