Sortilin as a new membrane inhibitor of EGFR trafficking for overcoming resistance to EGFR inhibitors in non-small cell lung cancer
Non-small cell lung cancer (NSCLC) accounts for about 85% of lung cancer, which is the most common and leading cause of cancer-related death in the United States and worldwide (1,2). The clinical benefit of cytotoxic chemotherapy doublets reached a plateau of median overall survival of 7–8 months and a 5-year survival rate of <5% in molecularly unselected patients with metastatic NSCLC in 2000 (3). Advances in cancer biology research and genomics technology enable the current era of precision oncology to treat an individual’s cancer based on the unique genetic and immune biomarkers (4,5). Drugs targeting the epidermal growth factor receptor (EGFR), by either small-molecule tyrosine kinase inhibitors (TKIs) or neutralizing monoclonal antibodies (mAbs), are by far the most successful molecularly targeted therapy developed in NSCLC, which have significantly improved the overall survival over chemotherapy in patients with metastatic NSCLC since 2004 (6).
EGFR is expressed on the surface of epithelial carcinoma cells and plays an essential role in the tumorigenesis, proliferation, differentiation, migration, anti-apoptosis, angiogenesis, and metastasis (7). Several mechanisms, such as EGFR overexpression, autocrine ligand stimulation, or constitutively activated mutant receptor, can lead to abnormal receptor activation, resulting in a variety of pathophysiological diseases and promoting oncogenesis or cancer development. In addition, EGFR could be transactivated in the absence of a specific ligand through G protein-coupled receptor activation. The presence of gain-of-function somatic mutations in the tyrosine kinase domain of the EGFR gene in NSCLC tumors defines the first molecular subset of 10–15% of Caucasian patients and 30–40% of East Asian patients who have a response rate of 60–80%, a median progression-free survival of 9–19 months and a median overall survival of 18–36 months to first-line EGFR TKIs (i.e., erlotinib, gefitinib, afatinib, dacomitinib, osimertinib) (6). The clinical benefit of EGFR TKIs in EGFR-mutant NSCLC cells is mainly due to its cytotoxic effects by induction of apoptosis (8,9), while their mechanism of action in EGFR-wild type NSCLC cells is mainly cytostatic by induction of G1 arrest and inhibition of tumor growth (10,11). However, the magnitude of tumor regression is often variable and transient (12). Mechanisms of primary resistance to EGFR-TKIs include in-frame insertion mutation in EGFR exon 20, de novo EGFR T790M mutation, KRAS mutations, loss of PTEN, and MET pro-oncogene amplification. Almost all patients with EGFR-mutant NSCLC eventually develop acquired resistance to the EGFR TKIs, which include the detection of a second-site mutation in the EGFR gene (such as T790M, V769M, L747S) (~50% of cases), MET pro-oncogene amplification (20%), or other molecular mechanisms such as upregulation of bypass RTK function (13). For patients with metastatic squamous NSCLC with EGFR-wild type gene, second generation EGFR TKI afatinib and second generation EGFR mAb necitumumab in combination with gemcitabine and cisplatin have been approved for second- and first-line treatment, respectively (14,15). Many strategies have been attempted to overcome primary and acquired resistance to EGFR-targeting therapy (6). One of the novel strategies for overcoming resistance to EGFR inhibitors is to inhibit EGFR trafficking as shown in this referenced paper (16).
EGFR functions as a receptor tyrosine kinase (RTK) localized on the plasma membrane with a transmembrane domain and is activated upon extracellular ligand binding to transduce information from the microenvironment into the cell and activate homeostatic downstream signaling pathways (6,7). EGFR consists of an extracellular domain (ECD), a transmembrane lipophilic segment, and an intracellular domain (ICD) containing a tyrosine kinase domain. At least six EGFR ligands have been identified, including epidermal growth factor (EGF), heparin binding-EGF, amphiregulin (ARG), and transforming growth factor (TGF)-α. Upon the ligand binding to the ECD, dimerization of the transmembrane EGFR induces autophosphorylation at distinct tyrosine residues of ICD, mediating several major signaling pathways, including the RAS/RAF/MEK/ERK pathway, PI3K/AKT/mTOR, and JAK/STAT pathways, for cell proliferation, survival, invasion, migration, anti-apoptosis, and pro-angiogenesis. Internalization and degradation of EGFR after ligand binding limits the intensity of proliferative signaling, which is a crucial step for signal termination and maintenance of cell integrity. In cancer cells, dysregulation of EGFR trafficking contributed to uncontrolled cell proliferation and survival. However, the selection of additional therapies increasingly depends on the molecular composition of the tumor and the mechanism of resistance.
Sortilin, encoded by the SORT1 gene on chromosome 1 at the band 1p13.3 in human, is a type I membrane glycoprotein in the vacuolar protein sorting 10 (VPS10) protein family of sorting receptors (17). Sortilin is ubiquitously expressed in many human tissues and shuttles between the plasma membrane, subcellular compartments such as endosomes, lysosomes, and the trans-Golgi network (TGN) (Figure 1). Sortilin acts as a multifaceted sorting receptor, sortilin facilitates the transportation of many intracellular proteins involved in many critical physiological processes such as lipid and glucose metabolism, neural development and cell death, as well as several major human diseases such as cardiovascular disease, Alzheimer’s disease, type 2 diabetes mellitus, and most recently cancer (19,20). Following their previous work showing sortilin is important for transporting and loading EGFR into extracellular vesicles via endocytosis (21), Al-Akhrass et al. determined the role of sortilin in regulating EGFR intracellular trafficking in this paper (16). They showed that sortilin regulated EGFR activity by inhibiting its internalization from the plasma membrane, thereby limiting proliferative signaling driving tumor aggressiveness. Sortilin exhibits its inhibitory effect on EGFR via a ligand independent mechanism, i.e., an independent mechanism of EGF-induced EGFR phosphorylation and endocytosis. Loss of sortilin in tumor cells promoted cell proliferation and accelerated tumor growth by sustaining EGFR signaling on the cell surface. In lung cancer patients, sortilin expression was correlated with high pathologic grade and poor overall survival, especially in patients with high EGFR expression. Sortilin acts as a tumor suppressor inhibiting tumorigenesis in the EGFR-mutant lung cancers. In contrast, sortilin acts as an oncogene promoting malignant behavior in EGFR-wild type lung cancers (22).
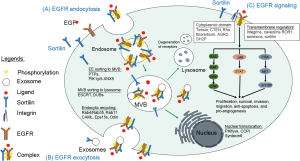
There are several clinical implications of this study. First, targeting EGFR trafficking by modulating sortilin expression is a novel strategy to overcome primary or acquired resistance to EGFR TKIs in EGFR-mutant NSCLC. Second, targeting EGFR trafficking by modulating sortilin expression might be particularly important for NSCLC and other types of EGFR-expressing tumors, such as glioblastomas, colorectal cancer, and head and neck cancers, that are not driven by the gain-of-function mutations in tyrosine kinase domains. Third, sortilin regulates ligand-independent EGFR or other RTK signaling which is important for regulating the tumor microenvironment, immunity, inflammation, and tissue repair (23). Nevertheless, there are several questions that remain to be answered before clinical translation. First, the prevalence of sortilin expression in EGFR-expressing NSCLC. Second, the development of therapeutics targeting sortilin.
It is worthy to mention that membrane proteins are an important class of proteome encoded by about 30% of the human protein coding genes (24) and represent about 70% of known clinical drug targets (25). Many of the membrane proteins have been explored as important targets for cancer biomarker discovery and drug development. Table 1 summarizes several key membrane-related proteins that have been associated with regulating EGFR trafficking through a variety of mechanisms, including (I) cytoplasmic regulators, such as tensin, C-terminal tensin-like (CTEN), Rho, thioredoxin (TRX), anterior gradient homolog 2 (AGR2), and Src homology 2 phosphotyrosine (SH2P); and (II) transmembrane regulators such as integrins, caveolins, RTK like orphan receptor 1 (ROR1), annexins, and sortilin. Further mechanistic studies are needed to elucidate the interaction between these membrane proteins in regulating the function of EGFR in the context of other RTKs that are involved in initiation and progression of lung adenocarcinoma and develop therapeutic strategies to improve the efficacy of EGFR inhibitors.
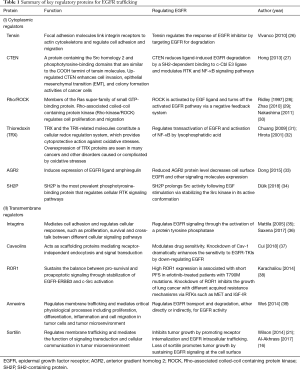
Full table
In conclusion, sortilin has been identified as a new negative membrane regulator for inhibiting EGFR intracellular trafficking in NSCLC. Sortilin expression is a favorable prognostic marker for patients with lung adenocarcinoma, independent of the mutation status in the EGFR tyrosine kinase domain. Further studies are needed to investigate the role of targeting sortilin and other EGFR-membrane associated proteins as a novel therapeutic strategy to improve EGFR-targeting therapy in NSCLC and other types of EGFR-expressing cancers.
Acknowledgements
Funding: This work was supported by “Novel Treatment Strategies for Adenocarcinomas” (UCD grant #49873) to T Li. Dr. Q Li was also supported by a research and training scholarship from Shanghai Jiaotong University Affiliated Sixth People’s Hospital.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524-48. [Crossref] [PubMed]
- Cetin K, Ettinger DS, Hei YJ, et al. Survival by histologic subtype in stage IV nonsmall cell lung cancer based on data from the Surveillance, Epidemiology and End Results Program. Clin Epidemiol 2011;3:139-48. [Crossref] [PubMed]
- Li T, Kung HJ, Mack PC, et al. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J Clin Oncol 2013;31:1039-49. [Crossref] [PubMed]
- Ma W, Gilligan BM, Yuan J, et al. Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy. J Hematol Oncol 2016;9:47. [Crossref] [PubMed]
- Rotow J, Bivona TG. Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer 2017;17:637-58. [Crossref] [PubMed]
- Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol 2003;21:2787-99. [Crossref] [PubMed]
- Sordella R, Bell DW, Haber DA, et al. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science 2004;305:1163-7. [Crossref] [PubMed]
- Tracy S, Mukohara T, Hansen M, et al. Gefitinib induces apoptosis in the EGFRL858R non-small-cell lung cancer cell line H3255. Cancer Res 2004;64:7241-4. [Crossref] [PubMed]
- Gandara DR, Gumerlock PH. Epidermal growth factor receptor tyrosine kinase inhibitors plus chemotherapy: case closed or is the jury still out? J Clin Oncol 2005;23:5856-8. [Crossref] [PubMed]
- Li T, Ling YH, Goldman ID, et al. Schedule-dependent cytotoxic synergism of pemetrexed and erlotinib in human non-small cell lung cancer cells. Clin Cancer Res 2007;13:3413-22. [Crossref] [PubMed]
- Bivona TG, Hieronymus H, Parker J, et al. FAS and NF-κB signalling modulate dependence of lung cancers on mutant EGFR. Nature 2011;471:523-6. [Crossref] [PubMed]
- Zhong WZ, Zhou Q, Wu YL. The resistance mechanisms and treatment strategies for EGFR-mutant advanced non-small-cell lung cancer. Oncotarget 2017;8:71358-70. [Crossref] [PubMed]
- Soria JC, Felip E, Cobo M, et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 2015;16:897-907. [Crossref] [PubMed]
- Thatcher N, Hirsch FR, Luft AV, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol 2015;16:763-74. [Crossref] [PubMed]
- Al-Akhrass H, Naves T, Vincent F, et al. Sortilin limits EGFR signaling by promoting its internalization in lung cancer. Nat Commun 2017;8:1182. [Crossref] [PubMed]
- Mazella J, Zsurger N, Navarro V, et al. The 100-kDa neurotensin receptor is gp95/sortilin, a non-G-protein-coupled receptor. J Biol Chem 1998;273:26273-6. [Crossref] [PubMed]
- Tomas A, Futter CE, Eden ER. EGF receptor trafficking: consequences for signaling and cancer. Trends Cell Biol 2014;24:26-34. [Crossref] [PubMed]
- Goettsch C, Kjolby M, Aikawa E. Sortilin and Its Multiple Roles in Cardiovascular and Metabolic Diseases. Arterioscler Thromb Vasc Biol 2018;38:19-25. [Crossref] [PubMed]
- Wilson CM, Naves T, Al Akhrass H, et al. A new role under sortilin's belt in cancer. Commun Integr Biol 2016;9. [Crossref] [PubMed]
- Wilson CM, Naves T, Vincent F, et al. Sortilin mediates the release and transfer of exosomes in concert with two tyrosine kinase receptors. J Cell Sci 2014;127:3983-97. [Crossref] [PubMed]
- Ghaemimanesh F, Ahmadian G, Talebi S, et al. The effect of sortilin silencing on ovarian carcinoma cells. Avicenna J Med Biotechnol 2014;6:169-77. [PubMed]
- Zaiss DMW, Gause WC, Osborne LC, et al. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity 2015;42:216-26. [Crossref] [PubMed]
- Ahram M, Litou ZI, Fang R, et al. Estimation of membrane proteins in the human proteome. In Silico Biol 2006;6:379-86. [PubMed]
- Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov 2002;1:727-30. [Crossref] [PubMed]
- Vivanco I, Rohle D, Versele M, et al. The phosphatase and tensin homolog regulates epidermal growth factor receptor (EGFR) inhibitor response by targeting EGFR for degradation. Proc Natl Acad Sci U S A 2010;107:6459-64. [Crossref] [PubMed]
- Hong SY, Shih YP, Li T, et al. CTEN prolongs signaling by EGFR through reducing its ligand-induced degradation. Cancer Res 2013;73:5266-76. [Crossref] [PubMed]
- Ridley AJ. The GTP-binding protein Rho. Int J Biochem Cell Biol 1997;29:1225-9. [Crossref] [PubMed]
- Zhao TT, Le Francois BG, Goss G, et al. Lovastatin inhibits EGFR dimerization and AKT activation in squamous cell carcinoma cells: potential regulation by targeting rho proteins. Oncogene 2010;29:4682-92. [Crossref] [PubMed]
- Nakashima M, Adachi S, Yasuda I, et al. Inhibition of Rho-associated coiled-coil containing protein kinase enhances the activation of epidermal growth factor receptor in pancreatic cancer cells. Mol Cancer 2011;10:79. [Crossref] [PubMed]
- Chuang CY, Chang CH, Huang YL. Thioredoxin mediates remodeling factors of human bronchial epithelial cells upon interaction with house dust mite-stimulated eosinophils. Inhal Toxicol 2009;21:153-67. [Crossref] [PubMed]
- Hirota K, Murata M, Itoh T, Yodoi J, Fukuda K. An endogenous redox molecule, thioredoxin, regulates transactivation of epidermal growth factor receptor and activation of NF-kappaB by lysophosphatidic acid. FEBS Lett 2001;489:134-8. [Crossref] [PubMed]
- Dong A, Wodziak D, Lowe AW. Epidermal growth factor receptor (EGFR) signaling requires a specific endoplasmic reticulum thioredoxin for the post-translational control of receptor presentation to the cell surface. J Biol Chem 2015;290:8016-27. [Crossref] [PubMed]
- Dülk M, Szeder B, Glatz G, et al. EGF Regulates the Interaction of Tks4 with Src through Its SH2 and SH3 Domains. Biochemistry 2018;57:4186-96. [Crossref] [PubMed]
- Mattila E, Pellinen T, Nevo J, et al. Negative regulation of EGFR signalling through integrin-alpha1beta1-mediated activation of protein tyrosine phosphatase TCPTP. Nat Cell Biol 2005;7:78-85. [Crossref] [PubMed]
- Saxena M, Liu S, Yang B, et al. EGFR and HER2 activate rigidity sensing only on rigid matrices. Nat Mater 2017;16:775-81. [PubMed]
- Cui Y, Zhu T, Song X, et al. Downregulation of caveolin-1 increased EGFR-TKIs sensitivity in lung adenocarcinoma cell line with EGFR mutation. Biochem Biophys Res Commun 2018;495:733-9. [Crossref] [PubMed]
- Karachaliou N, Gimenez-Capitan A, Drozdowskyj A, et al. ROR1 as a novel therapeutic target for EGFR-mutant non-small-cell lung cancer patients with the EGFR T790M mutation. Transl Lung Cancer Res 2014;3:122-30. [PubMed]
- Woś M, Bandorowicz-Pikula J. Participation of annexins in endocytosis and EGFR-mediated signal transduction. Postepy Biochem 2014;60:55-61. [PubMed]


