Characterization of primary symptoms leading to Chinese patients presenting at hospital with suspected obstructive sleep apnea
Introduction
Obstructive sleep apnea (OSA) is highly common in the general population and may affect 9% to 17% of adults with higher rates in men (1). Recurring episodes of apnea during sleep is the central pathological component of OSA, but symptoms and associated illnesses vary among individual patients. Symptoms typically include loud snoring, witnessed episodes of apnea during sleep, gasping and daytime sleepiness. OSA also has significant comorbidity with hypertension, cardiovascular diseases, stroke and diabetes as well as other illnesses (2). Effective treatment, even for mild OSA patients, may ameliorate comorbid conditions.
Though symptoms vary among OSA patients, there are two primary reasons for which potential patients present to a sleep laboratory for polysomnography (PSG). Patients may be self-aware of symptoms and seek medical assistance or they may be referred by a physician who notes OSA related symptoms in patients who have presented with hypertension, heart diseases, stroke or other comorbidities. Patient’s self-awareness may largely arise from public awareness programs whereas referral likely reflects the cognizance of OSA by physicians. Clarifying these issues would be valuable for strategizing for the development and improvement of respiratory sleep medicine. For example, a low number of referred OSA patients would suggest that we may need to increase education related to respiratory sleep medicine knowledge to physicians. Further, these two patient pools could influence the evaluation and characterization of OSA as well as differentially skew data regarding OSA prevalence in hospital based studies. For instance, if the data include fewer referred OSA patients, the average age of the pool may be younger. In the present investigation, we used a case series approach to identify the primary symptoms that lead to patients presenting at our sleep medicine center in China.
However, many complicated factors may impact the characteristics of OSA prevalence across races and countries, including biological and socioeconomic factors (3). In the current study, we found a particularly young average age and high prevalence of severe OSA in patients with suspected OSA. To further evaluate potential differences specific to Chinese OSA patients, we therefore compared our results to those in studies of populations of other countries in order to assess potential demographic differences in age and prevalence of severe OSA across nationalities.
Methods
Subjects
The data were collected from October, 2011 to December, 2011 at the Sleep Medicine Center of West China Hospital, Sichuan University (Chengdu, China). During this three months period, 350 (302 males and 48 females, average age 42.9±11.0 years old) consecutive patients with suspected OSA were referred to take an overnight PSG. Data from a subset of these patients, as identified below, were used in the study. This study was approved by the Research Ethics Board of the West China Hospital of Sichuan University.
Clinical data collection
Prior to PSG recording, clinic data collection mainly included three steps. First, we recorded the information concerning PSG prescribed by the physicians across departments. The results were summarized in Table 1. Second, routine information was collected for all patients who underwent PSG in our sleep medicine center. These included checklists for general information (e.g., age and gender, totally seven items), symptoms (e.g., snoring, observed apnea and leg movement, choking and morning headache, totally 13 items), comorbidities (e.g., hypertension and heart disease, totally ten items), lifestyle (smoking and alcohol, totally five items) and a checklist for physical examination (height, body weight, blood pressure and pharyngeal tonsils, totally ten items). The Epworth daytime sleepiness scale (ESS) was also evaluated. However, we only present some of most important data in Table 2.
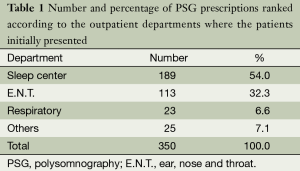
Full table
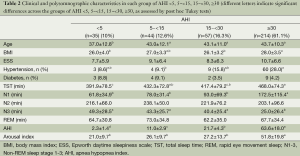
Full table
Third, we specifically identified the primary symptoms that led to the patients presenting at the sleep medicine center for PSG for this study. In this step, we attempted to classify the primary symptoms as a distinct category. For example, patients presenting with a complaint of loud snoring alone with no other symptoms were classified as “snoring”. However, many patients might have multiple symptoms. We carefully investigated what the single primary issue that led to present the hospital. For example, if a patient had symptoms of snoring, observed episodes of apnea, daytime sleepiness and hypertension, he might answered that “my wife observed the apnea and pushed me to come to the hospital”, he then would be classified as “observed apnea”; if his answer was daytime sleepiness, he would be recognized into that category; if he stated that due to “I have suffered from hypertension and PSG was prescribed by my doctor of internal medicine”, he would be categorized as “hypertension”. In fact, most patients could clearly identify as one of them. However, if the patients presented with insomnia, regardless of whether the patients were suspected of OSA, they would be placed into an insomnia category in our registration system and excluded from the investigation.
Overnight PSG
The diagnosis of OSA was established by a standard overnight PSG. Overnight PSG consisted of continuous recordings from six electroencephalographic leads (F3-A2, F4-C1, C3-A2, C4-A1, O1-A2, O2-A1, international 10-20 system), two electrooculographic leads (ROCA1, LOC-A2), four electromyography leads (two submental and bilateral tibialis anterior), thermistors for nasal and oral airflow, strain gauges for thoracic and abdominal excursion, finger pulse oximetry, and electrocardiography. Thirty-second epochs were analyzed and sleep stages were scored according to the international criteria of American Academy of Sleep Medicine. An apnea was defined as more than 90% reduction in airflow for at least 10 s; hypopnea as 50% or more reduction of airflow for at least 10 s associated with 3% or more reduction in oxygen saturation.
Statistical analysis
Comparisons between both groups of patients were performed using independent t-tests and the post hoc Tukey test for normally and abnormally distributed data, respectively. Chi-square analyses were used for categorical data (hypertension and diabetes). Statistical significance was defined using a P value of 0.05. Statistical analyses were performed with Statistic Package for Social Science (SPSS) for Windows software (version 17.0). Results are shown as mean ± standard deviation (SD).
Results
As shown in Table 1, of the 350 patients, 54% and 32% (total 86%) patients directly came to the outpatient clinics of our sleep medicine center and ear, nose and throat (E.N.T.) department, respectively. Most had clear complaints of apnea and snoring. Another 7% presented at the outpatient clinic of respiratory medicine and the remaining 7% presented at the outpatient clinics in other departments, such as internal medicine, neurology, Chinese sleep medicine, etc.
Of the consecutive 350 patients with clinic suspicion of OSA, we identified 24 different primary symptoms that led to patients presenting at the clinic. As shown in Figure 1, the most prevalent primary symptoms were witnessed apnea (32.9%), snoring alone (28.9%), and choking/gasping (13.7%). Figure 1 also presents eight primary symptoms with less percentages of occurrence [e.g., daytime sleepiness (4.9%), etc.], and rest of 13 primary symptoms were classified as “other” (6.3%) presented in Figure 1. The 13 symptoms included pharynx choking (n=3), bruxism (n=2), nose bleeds (n=3), numbness in hands and feet (n=2), tonsillitis (n=3), irritability (n=1), polycythemia (n=1), chronic bronchitis (n=1), excessive dreaming (n=1), regurgitation of food (n=1), rhinitis (n=2), turbinate hypertrophy (n=1), and pleurisy (n=1).
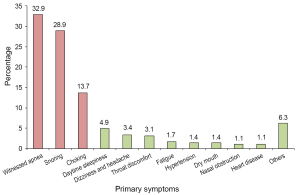
Table 3 presents the percentage and apnea hypopnea index (AHI) mean values for AHI 5~<15 (mild), 15~<30 (moderate), ≥30 (severe) for each grouping of primary symptoms. Considering the distribution of patients’ number in the different primary symptoms, we divided the patients into five different groups according to primary symptoms as shown in Figure 1. Using severe OSA criteria (AHI ≥30), the rankings of OSA percentage across symptom groups were daytime sleepiness (88%) > witnessed apnea (69%) > choking (58%) = snoring (55%) = rest (54%). The AHI in the daytime sleepiness group was significantly greater than that found in the other four symptom groups.
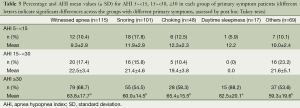
Full table
As shown in Table 2, 12.6%, 16.3% and 61.1% of the patients had AHI between 5~<15, 15~<30, and greater than 30, respectively. Compared to patients with AHI <5, those with mild, moderate and severe OSA, had significantly greater ages. Patients with severe OSA had greater BMI than those with AHI <5 and moderate OSA, higher likelihood of hypertension (28%) than those with mild OSA (9%). With regard to sleep parameters, patients with severe OSA had consistently greater arousal index and time spent in N1, and fewer amount of N3 time, compared to those with AHI <5, mild and moderate OSA.
Discussion
Immediate data interpretation
Of the 350 patients who presented at our sleep medicine center to take an overnight study for OSA diagnosis, nearly 80% had self-aware symptoms of snoring alone, witnessed apnea, choking/gasping, daytime sleepiness and others (Figure 1). Additionally, 86% of patients presented at outpatient the clinics of the sleep medicine center (54%) and ENT (32%) had clear concerns for OSA; some patients seen at the ENT clinic had throat discomfort, nasal obstruction and other symptoms, and were referred due to their ENT physician’s concerns regarding potential OSA. However, only 2.6% of patients with primary symptoms of hypertension and heart diseases were referred for OSA screening. Together, these results indicate that the majority of the patients were aware of their symptoms and presented at the hospital for the specific purpose of solving their sleep breathing problems. China has no established system for family physicians; thus, patients directly go to hospitals and departments based on their understanding of their own medical needs. Additionally, only medium and large sized hospitals have sleep disorder centers capable of conducting overnight PSG studies. The fact that witnessed apnea was the number one primary symptom (33%) that led to patients presenting in the hospital suggests that public education efforts for OSA aimed at middle aged spouses, particularly to women (the results showed that patients were 86% male, age was 43±11 years old, and most were married) is likely an important approach to enhance respiratory sleep medicine in China. In addition, for witnessed apnea and snoring alone, the percentage for severe OSA (AHI greater than 30) was higher among individuals with the complaint of witnessed apnea (69%) compared to those with the complaint of loud snoring alone (54%), but the differences were not as great as we expected. This suggests, for more severe OSA patients, that the apnea might not be noted by bed partners; thus, routine PSG screen for OSA is still necessary for loud snoring individuals.
Characterization of OSA in East Asians compared to other regions in the literature
In this case series of 350 patients, mean age was 43±11 years and the prevalent rate of severe OSA (AHI ≥30) was 61%. Among diagnosed severe OSA patients (n=214), age was 43.7±10.3 years old, BMI was 28±3.5 kg/m2, AHI was 54±18, hypertension was 28%, and sleep architecture was characterized by a nearly threefold increase in N1 time and more than a twofold decrease in N3 time. Those are likely the most important parameters to evaluate OSA prevalence and severity.
By comparing our data to that of published studies, we noted that the age of patients appears to be younger and the prevalence of severe OSA appears to be higher in the countries of East Asian compared to other regions. In order assess these potential differences in a more detailed manner, through an extensive literature review, we obtained, through PubMed, 470 papers based on the search terms “sleep apnea and consecutive and age” that were published during the recent ten years. We eliminated non-case series and papers that focused on children, a specific male or female sample, a specific illness, a specific age, a community based study and so on. We found 28 cases serial studies conducted in 17 different countries, which we believe are comparable to our study. In Figure 2, including the results of current study (labeled as “Current”), we present average age in patients with suspected OSA from 29 reports (left panel) and prevalence of severe OSA (AHI >30) in 11 reports (right panel). As clearly reflect in Figure 2, eight studies with the youngest age (from left to right) were conducted in four different countries in Eastern Asia [South Korea, 42 years (4); Singapore, 43 and 45 years (5,6); China, 43 (current study) and 45 years (7); Japan, 46, 47 and 48 years (8-10)]. By comparison, in the remaining 21 studies with ages above 48 years, only three were reported from Japan (49, 50 and 50 years old) (12,15,16) and rest were reported from 13 different countries in North America (13,18,20,25), South America (23), Europe (17,21,22,24,26-31) and the Middle East (11,14,19). The average age in all 11 reports from the countries of Eastern Asia was 46 (range from 42 to 50) years, whereas it was 52 (range from 48 to 57) years in the 18 reports from the other countries. We found only ten papers that clearly indicated the prevalence of severe OSA prevalence among those we examined. As shown in the right panel of Figure 2, the three highest percentages were reported in the current study conducted in China (61%) and in two studies conducted in Japan (58%, 45%) (15,32), no studies reported the percentage above 45% (range, 45-16%) in the remaining 8 papers conduced in other regions (11,13,17,21,26,28,33,34). Even using the criteria of AHI >40, the prevalence still reached 44% in one study in China (7). Review of these reports plus our current study suggest that the age onset of illness is considerably younger and that prevalence is remarkably higher for OSA in East Asia, compared to North America, South America and Europe.
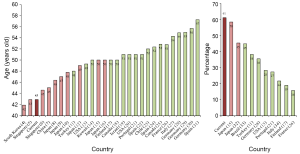
OSA patients in East Asia may also have greater AHI, lower BMI, more severe objective sleep changes, and less hypertension comorbidity compared to those in North America, South America and Europe. For example, for severity of OSA, the current work shows that the means of AHI was 64/h for severe OSA patients, whereas mean AHI was 43/h for severe OSA patients in a similar study in Europe (34). Regarding BMI, the current investigation shows a mean of BMI of 28 kg/m2, which is close to that in similar studies in the mainland of China (27 kg/m2) (7), Singapore (28 kg/m2) (5) and Japan (25-28 kg/m2) (9,10,12,15). In contrast, BMI appeared to be markedly higher in the USA (34-37 kg/m2) (13,20), Italy (36 kg/m2) (27), Spain (30-32 kg/m2) (22,24,31), Germany (32 kg/m2) (29,30), Portugal (31 kg/m2) (21), and in Canada (32 kg/m2) (25). For hypertension in severe OSA patients, we found comorbidity was 28%, but studies report comorbidity of 67% in Italy (34), 44% in USA (13) and 41% in Turkey (11).
For mild, moderate and severe OSA, we found significant increases in the number of brief arousals and N1 time, and a decrease in N3 time, compared to patients with AHI less than five. Similar disruptions in objective sleep measures have been consistently reported in OSA studies, but the severity appears to be less than the level we found. For example, we found a threefold increase in N1 time and a nearly twofold decrease in N3 time in the severe OSA group relative to the group with AHI less than five. Previous studies have not reported similar levels of changes in sleep.
Biological or socioeconomic issues?
Through extensive literature review (described in the Results section) of currently available case series studies, our results suggest that OSA patients in countries of East Asia are characterized by higher prevalence, more severe apnea, younger age, poorer sleep quality, but less obesity and less comorbidity with hypertension, relative to countries in North America, South America and Europe. As extensively reviewed by Villaneuva et al. (3), we believe there are at least two major factors which may impact the prevalence of OSA in East Asian populations.
First, narrower upper airways, which are related to congenital ethnic characteristics of the skeletal craniofacial structure of East Asians compared to Caucasians (3), possibly lead to OSA patients in this population having higher prevalence, more severe symptoms and earlier age of onset, compared to other races. For the consistently large differences in prevalence and average age in the countries of East Asia compared to the countries of North America, South America and Europe, we believe that this biological issue may play the major role in differences across races. In particular, the current investigation and previous studies showed that mean patient BMI was 25-29 kg/m2 in East Asians (5,7,9,10,12,15), whereas mean BMI was 30-37 kg/m2 in Caucasians (13,20-22,24,25,27,29-31). The characteristic of relatively low BMI may implicate that local anatomic factors may play more important role than systematic factors in the high prevalence of OSA in East Asians.
Secondly, complicated differences in socioeconomic levels and both general and medical education levels across institutes, societies, regions, and countries would also significantly affect the evaluation of prevalence in case series study. For example, cardiovascular physicians knowledgeable of sleep breathing disorders who regularly prescribe PSG examinations may significantly change the composition of data collected at a single sleep medicine center, driving an increased average age for hospital based studies. It is possible that more developed countries may have better training in sleep medicine, and thus physicians may pay more attention to OSA comorbidities which could lead to differences in the constitution of the patient pool. In addition, the fact of underdevelopment of sleep medicine in China may drive greater percentage of severe OSA patients to seek medical assistance. As we can see in the left panel of Figure 2, among the five countries of East Asia, Japan may be the most developed country and has the oldest reported ages in three studies collected in that country.
We believe that identifying the factors underlying this difference is an important issue that needs to be addressed in future research. However, we are aware that the current work does not distinguish many complicated factors underlying the characteristics of prevalence of OSA in hospital based studies. For example, the study was carried out in a single institute and did not reflect the characteristics of the prevalence of OSA in the entire nation of China. Secondly, for insomnia patients, snoring-associated issues are not normally the primary factors driving patients to seek medical assistance. The current study is focused on the characterization of primary symptoms in possible OSA patients. Therefore we did not include insomnia complaints into this study, though the exclusion of insomnia may have resulted in exclusion of some OSA patients, particularly elderly patients.
Conclusions
In summary, the current results and existing literature suggest that OSA in East Asian countries may have higher prevalence, younger onset age, greater severity and poorer sleep quality, but with less obesity and less comorbidity with hypertension, relative to OSA in Caucasians and other races.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81170072, 81328010) and by the Sino-German Joint Center for Sleep Medicine (GZ538).
Disclosure: This was not an industry supported study. The authors have indicated no financial conflicts of interest.
References
- Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea?: The Rational Clinical Examination systematic review. JAMA 2013;310:731-41. [PubMed]
- Hamilton GS, Naughton MT. Impact of obstructive sleep apnoea on diabetes and cardiovascular disease. Med J Aust 2013;199:S27-30. [PubMed]
- Villaneuva AT, Buchanan PR, Yee BJ, et al. Ethnicity and obstructive sleep apnoea. Sleep Med Rev 2005;9:419-36. [PubMed]
- Lee SJ, Kang HW, Lee LH. The relationship between the Epworth Sleepiness Scale and polysomnographic parameters in obstructive sleep apnea patients. Eur Arch Otorhinolaryngol 2012;269:1143-7. [PubMed]
- Thong JF, Pang KP. Clinical parameters in obstructive sleep apnea: are there any correlations? J Otolaryngol Head Neck Surg 2008;37:894-900. [PubMed]
- Leng PH, Mosharraf-Hossain AK, Chan YH, et al. The clinical predictors of hypertension and sleepiness in an Asian population with sleep-disordered breathing. Ann Acad Med Singapore 2006;35:6-10. [PubMed]
- Chen R, Xiong KP, Lian YX, et al. Daytime sleepiness and its determining factors in Chinese obstructive sleep apnea patients. Sleep Breath 2011;15:129-35. [PubMed]
- Oga T, Chin K, Tabuchi A, et al. Effects of obstructive sleep apnea with intermittent hypoxia on platelet aggregability. J Atheroscler Thromb 2009;16:862-9. [PubMed]
- Nakano H, Hirayama K, Sadamitsu Y, et al. Mean tracheal sound energy during sleep is related to daytime blood pressure. Sleep 2013;36:1361-7. [PubMed]
- Yamashiro Y, Kryger M. Is laryngeal descent associated with increased risk for obstructive sleep apnea? Chest 2012;141:1407-13. [PubMed]
- Kepez A, Niksarlıoğlu EY, Hazırolan T, et al. Evaluation of association between obstructive sleep apnea and coronary risk scores predicted by tomographic coronary calcium scoring in asymptomatic patients. Anadolu Kardiyol Derg 2011;11:428-35. [PubMed]
- Hayano J, Watanabe E, Saito Y, et al. Screening for obstructive sleep apnea by cyclic variation of heart rate. Circ Arrhythm Electrophysiol 2011;4:64-72. [PubMed]
- Hudgel DW, Lamerato LE, Jacobsen GR, et al. Assessment of multiple health risks in a single obstructive sleep apnea population. J Clin Sleep Med 2012;8:9-18. [PubMed]
- Al-Alawi A, Mulgrew A, Tench E, et al. Prevalence, risk factors and impact on daytime sleepiness and hypertension of periodic leg movements with arousals in patients with obstructive sleep apnea. J Clin Sleep Med 2006;2:281-7. [PubMed]
- Kubota Y, Nakayama H, Takada T, et al. Facial axis angle as a risk factor for obstructive sleep apnea. Intern Med 2005;44:805-10. [PubMed]
- Kato T, Mikami A, Sugita H, et al. Negative association between self-reported jaw symptoms and apnea-hypopnea index in patients with symptoms of obstructive sleep apnea syndrome: a pilot study. Sleep Breath 2013;17:373-9. [PubMed]
- Kreivi HR, Salmi T, Maasilta P, et al. Screening of snoring with an MP3 recorder. Sleep Breath 2013;17:77-84. [PubMed]
- Pereira EJ, Driver HS, Stewart SC, et al. Comparing a combination of validated questionnaires and level III portable monitor with polysomnography to diagnose and exclude sleep apnea. J Clin Sleep Med 2013;9:1259-66. [PubMed]
- Oksenberg A, Froom P, Melamed S. Dry mouth upon awakening in obstructive sleep apnea. J Sleep Res 2006;15:317-20. [PubMed]
- Reyes-Zúñiga M, Castorena-Maldonado A, Carrillo-Alduenda JL, et al. Anxiety and depression symptoms in patients with sleep-disordered breathing. Open Respir Med J 2012;6:97-103. [PubMed]
- Vaz AP, Drummond M, Mota PC, et al. Translation of Berlin Questionnaire to Portuguese language and its application in OSA identification in a sleep disordered breathing clinic. Rev Port Pneumol 2011;17:59-65. [PubMed]
- Barceló X, Mirapeix RM, Bugés J, et al. Oropharyngeal examination to predict sleep apnea severity. Arch Otolaryngol Head Neck Surg 2011;137:990-6. [PubMed]
- Saldías PF, Jorquera AJ, Díaz PO. Predictive value of clinical features and nocturnal oximetry for the detection of obstructive sleep apnea syndrome. Rev Med Chil 2010;138:941-50. [PubMed]
- Candela A, Hernández L, Asensio S, et al. Validation of a respiratory polygraphy system in the diagnosis of sleep apnea syndrome. Arch Bronconeumol 2005;41:71-7. [PubMed]
- Driver HS, Pereira EJ, Bjerring K, et al. Validation of the MediByte® type 3 portable monitor compared with polysomnography for screening of obstructive sleep apnea. Can Respir J 2011;18:137-43. [PubMed]
- Chambe J, Laib S, Hubbard J, et al. Floppy eyelid syndrome is associated with obstructive sleep apnoea: a prospective study on 127 patients. J Sleep Res 2012;21:308-15. [PubMed]
- Olmetti F, La Rovere MT, Robbi E, et al. Nocturnal cardiac arrhythmia in patients with obstructive sleep apnea. Sleep Med 2008;9:475-80. [PubMed]
- Lentini S, Manka R, Scholtyssek S, et al. Creatine phosphokinase elevation in obstructive sleep apnea syndrome: an unknown association? Chest 2006;129:88-94. [PubMed]
- Böhning N, Zucchini W, Hörstmeier O, et al. Sensitivity and specificity of telemedicine-based long-term pulse-oximetry in comparison with cardiorespiratory polygraphy and polysomnography in patients with obstructive sleep apnoea syndrome. J Telemed Telecare 2011;17:15-9. [PubMed]
- Hübner RH, El Mokhtari NE, Freitag S, et al. NT-proBNP is not elevated in patients with obstructive sleep apnoea. Respir Med 2008;102:134-42. [PubMed]
- Novoa MT, Fernández AA, Perez MT, et al. Metabolic syndrome and its components in patients with sleep apnea syndrome. An Sist Sanit Navar 2011;34:363-72. [PubMed]
- Yamauchi M, Nakano H, Maekawa J, et al. Oxidative stress in obstructive sleep apnea. Chest 2005;127:1674-9. [PubMed]
- Dominici M, Gomes Mda M. Obstructive sleep apnea (OSA) and depressive symptoms. Arq Neuropsiquiatr 2009;67:35-9. [PubMed]
- Del Ben M, Fabiani M, Loffredo L, et al. Oxidative stress mediated arterial dysfunction in patients with obstructive sleep apnoea and the effect of continuous positive airway pressure treatment. BMC Pulm Med 2012;12:36. [PubMed]


