MALDI-TOF MS versus VITEK 2 ANC card for identification of anaerobic bacteria
Introduction
Anaerobic bacteria are a significant component of human mucous membranes bacterial flora, and often are the causative agent of respiratory, gastrointestinal and female genital tract infections and bacteremia. Anaerobes that combine with aerobic bacteria can cause serious mixed infections, and they are frequently overlooked. Rapid and accurate identification of anaerobes play an important role in timely and appropriate treatments.
Conventional identification of anaerobes has long been mainly based on the detection of phenotypic characteristics, such as Gram staining, colony morphology, microscopic examination, differential growth on selective media and various manual biochemical tests. Most of these conventional methods are laborious and time-consuming processes. While the development and popularization of automated and semi-automated systems continue for the identification of isolates, clinical application studies using these systems to identify anaerobes are limited. This is especially the case for the newly developed Vitek 2 anaerobe and Corynebacterium (ANC) card (bioMérieux, France) and the matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) system.
To expand the capabilities for identification of corynebacteria and anaerobic species on the Vitek 2 system, bioMérieux, Inc. has developed the new ANC card. Previous studies have confirmed that the Vitek 2 ANC card is a simple, rapid, and satisfactory method for the identification of anaerobes in a clinical microbiology laboratory (1-3). However, less information is provided in evaluating the Vitek 2 ANC card comparing to other identification systems (4). The ANC card has a database that includes 49 taxa of anaerobic bacteria belonging to the genera Actinomyces, Bacteroides, Bifidobacterium, Clostridium, Collinsella, Eggerthella, Eubacterium, Finegoldia, Fusobacterium, Lactobacillus, Parabacteroides, Parvimonas, Peptoniphilus, Peptostreptococcus, Prevotella, Propionibacterium, and Veillonella. Bifidobacterium spp. and Veillonella spp. are identified only at the genus level in this system. Identification is accomplished within approximately 6 h incubation time using a 64 microwell card that contains dehydrated biochemical substrates.
MALDI-TOF MS technology for the identification of bacteria is now gaining increased attention due to its accurate, inexpensive and rapid performance efficiencies (5-9). This technique is a soft ionization method, which allows desorption of peptides/proteins from both whole different cultured bacteria and crude bacterial extracts (10). Identification is based on the comparison of the tested isolate mass spectrum to a reference database. Bruker MS and Shimadzu MS, two types of MALDI-TOF MS systems, were frequently studied for anaerobic bacteria identification, proving varied rates of identification for anaerobes (11-14). The majority of previous studies have compared MALDI-TOF identification with conventional identification or reference standard methods simply for the identification of anaerobic bacteria (15-21).
In this study, we evaluated the Vitek MS and the Vitek 2 ANC card for the identification of most common clinical anaerobic isolates. 16S rRNA gene sequencing was used as a reference method (22).
Materials and methods
Bacterial strains and culture condition
A total of 50 fresh and frozen anaerobic clinical isolates comprising ten different genera and 14 species were included in the study. All isolates were recovered from routine examination of clinical specimens submitted to the First Affiliated Hospital of Nanjing Medical University. The five reference strains, Bacteroides fragilis ATCC 25285, Clostridium difficile ATCC 43255, Propionibacterium acnes ATCC 11827, Propionibacterium acnes ATCC 6919 and Lactobacillus acidophilus ATCC 4356, were tested routinely. Prior to testing, all strains were subcultured twice onto Columbia blood agar (bioMérieux, Shanghai, China) and incubated in an anaerobic atmosphere produced by GENbag (bioMérieux, Shanghai, China) for 48 h at 35 °C.
Vitek 2 ANC card
Bacterial colonies were suspended in 0.45% sodium chloride with a turbidity of 2.7-3.3 McFarland. Inoculums were then introduced into an ANC card in the Vitek 2 Compact automated identification system and incubated for approximately 6 h. Through the three additional tests of Gram staining, cell morphology, and aerotolerance testing, the Vitek 2 system deduced interpretations for final identifications. Isolates initially resulting in no identification were retested.
MALDI-TOF MS system
Vitek MS is an automated microbial identification system based on MALDI-TOF technology. Each isolate was directly smeared onto a disposable target slide and then covered by a small drop of matrix solution (Vitek MS-CHCA) and air dried. The loaded slide was then inserted into the Vitek MS system. The quality standard performed on each group was a spot of E. coli ATCC 8739. Microbial identification is achieved by obtaining a composite mass spectrum using MALDI-TOF technology and comparing the sample spectra to the reference spectra contained within the Vitek MS version 2.0 database.
16S rRNA gene sequencing
When discrepancies in identification were observed between the VITEK 2 ANC card and the VITEK MS system, or no identification achieved in both, 16S rRNA gene sequencing was used to confirm the result. Bacterial DNA was extracted using the TIANamp Bacteria DNA kit (TIANGEN, Beijing, China), and the sequencing reactions were performed with a 16S rDNA Bacterial Identification PCR Kit (Takara, Dalian, China) according to the manufacturer’s instructions. PCR products were purified and sequenced by Genscript Corporation. The obtained 16S rRNA gene sequences were subjected to BLAST analysis against the NCBI nucleotide database.
Data analysis
Data obtained were classified into the following categories: (I) correct identification to the species level; (II) correct identification to the genus level, including a multiple choice within the same genus; (III) low discrimination, between two or more species, including the correct species requiring additional tests; (IV) no identification; and (V) misidentification, the final identification in which the genus or species were incorrect compared to that of the reference 16S rRNA gene sequence.
Results
In this study, five ATCC reference strains were selected for evaluating the accuracy of Vitek MS and Vitek 2 ANC card. All of these five reference strains were identified routinely and showed consistent results except for ATCC 4356 Lactobacillus acidophilus (Table 1). The Vitek MS cannot provide the accurate result of Lactobacillus acidophilus at the species level, but rather only provides a selection of low discrimination identification between Lactobacillus acidophilus and Lactobacillus gasseri, while ANC card can identify the Lactobacillus acidophilus accurately.

Full table
To further evaluate the capability of Vitek MS and Vitek 2 ANC card, we selected 50 clinical isolates from our clinical anaerobe bank. Each strain was identified with the two systems respectively. For the 50 isolates belonging to ten genera and 14 different species (Table 2), Vitek MS provided correct identification for 46 (92%) isolates to species level, 47 (94%) isolates to the genus level, but one (2%) with low discrimination, two (4%) with no identification, and one (2%) with misidentification. In comparison, the Vitek 2 ANC card achieved 43 (86%) correct identification to the species level, 47 (94%) correct identification to the genus level, three (6%) low discrimination, three (6%) no identification, and one (2%) misidentification.
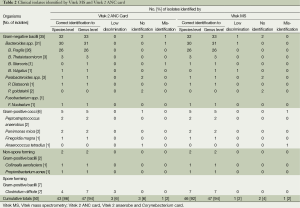
Full table
As seen in Table 3, there are eight discrepant results among the 50 pairs of identifications produced by Vitek MS and Vitek 2 ANC card. All of these discrepant strains were confirmed by 16S rRNA gene sequencing. One minor error by Vitek ANC card was an identification of Bacteroides stercoris instead of Bacteroides thetaiotaomicron. Bacteroides vulgatus displayed mixed genera identification of Bacteroides eggerthii and Bacteroides vulgatus by the Vitek MS system. The Vitek MS produced superior accurate results for identification of Clostridium difficile while the Vitek 2 ANC card performed low discrimination consisting of Clostridium spp. of three isolates of Clostridium difficile. C. difficile requires further tests of indole and lipase for distinction from C. bifermentans and C. sporogenes. Our results also revealed that Anaerococcus tetradius was not included in the Vitek MS database and was misidentified as Brevibacillus spp. The Vitek 2 ANC card cannot identify this species too. Moreover, two strains of Parabacteroides goldsteinii were not identified by either system.
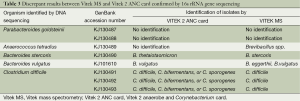
Full table
Discussion
Up to now, clinical anaerobe identification is still a time consuming and skilled process. There are a few automated systems or rapid identification reagents presently available for clinical laboratory use. The Vitek 2 ANC card and the Vitek MS are two new developed methods for anaerobe identification. Whether if they can improve clinical anaerobe identification? Which system should be better for clinical use? No study has answered these questions till now. In this study, five reference strains and 50 clinical isolates were selected to evaluate the two commercial automated systems in identifying anaerobic bacteria. From the results contained in Tables 1,2, we can see that both the ANC card and the Vitek MS can identify most of the reference strains and clinical isolates accurately. For the 50 clinical anaerobe strains, the two identification systems achieved the same percentage (94%) of correct identification at the genus level, which meets the requirements of clinical routine anaerobe identification. However, at the species level, Vitek MS got a considerably higher rate of identification accuracy of 92% compared to the Vitek ANC card of 86%. This data revealed that the Vitek MS is superior to the ANC card for species identification. Our results using Vitek MS are similar to a recent multi-centre evaluation of anaerobic bacteria, which exhibited 92.5% of correct identification at the genus level and 91.2% at the species level (15). Comparable results of Vitek ANC card were presented with 98.5% accuracy rate at genus level and 86.5% at species level by Francine Mory et al. (2). Moreover, both systems in our study generated excellent results in achieving 100% correct identification to the species level for Bacteroides fragilis.
However, three of seven of Clostridium difficile gave low-discrimination identification results at the species level when using ANC card. These findings are consistent with the previous work stating that the identification of Clostridia at the species level using the ANC card should be enhanced (1,2). But by contrast, 100% of Clostridium difficile isolates were identified correctly to the species level using Vitek MS. Among the 8 sequenced strains, there are four sequence results supporting the Vitek MS identification results, and only one isolate matches Vitek 2 ANC card identification result at species level, as shown in Table 3. Unsuccessful identification of Anaerococcus tetradius and Parabacteroides goldsteinii with both of the identification systems is due to the species not being included in the database. The reliability of the identification depends on the quality and composition of the reference spectra present in the database (3,11,23,24). The databases currently available for both systems need to be optimized with more spectra for certain genera and species and the very rare species need to be included to increase the identification capability of both automated systems.
As we known, only one anaerobe colony is required for identification using Vitek MS while bacterial turbidity need to be 2.7-3.3 McFarland using Vitek 2 ANC card. For slow growing of most anaerobic bacteria, the Vitek ANC card needs at least 24 h longer incubation time than the Vitek MS. On the other hand, all the isolates we studied can be identified on the first attempt by Vitek MS while more than 20% of the isolates had to be identified on a second or third attempt for discrepancies or no identification results were obtained by Vitek 2 ANC card. All these may increase the costs and extend the turnaround time of each test on the Vitek 2 ANC card additionally. A recent cost assessment study also demonstrated that the MALDI protocol provided identifications 1.45 days earlier on average and can reduce reagent and labor costs of identification by $102,424, or 56.9% less when compared with standard protocols (25). Besides the advantages mentioned before, Vitek MS is easy to perform even for a relatively inexperienced technician.
Overall, both the Vitek 2 ANC card and the Vitek MS can provide accurate identification of anaerobic bacteria and meet routine clinical requirements. However, the Vitek MS system is an easier, faster and cheaper method than the Vitek 2 ANC card for identification of most clinically important anaerobic bacteria, except for the initial cost of the Vitek MS instrument is significantly higher than the Vitek 2 instrument. Both our study and others found that there are still many bacteria not included in the databases currently available for both systems. Future developments of the databases to include an expanded number of new species and more robust mass spectra profiles for current species will greatly improve the performance and utility of these automated systems for bacterial identification.
Acknowledgements
We acknowledge bioMérieux for supplying Vitek MS and related regents. We are particularly grateful to Weiping Wang from Nanjing Jinling Hospital for her generous help. This study was funded by National Natural Science Foundation of China (No. 81371894, 81272324, 81201359, 81101322), Key Laboratory for Laboratory Medicine of Jiangsu Province of China (No. XK201114). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Rennie RP, Brosnikoff C, Turnbull L, et al. Multicenter evaluation of the Vitek 2 anaerobe and Corynebacterium identification card. J Clin Microbiol 2008;46:2646-51. [PubMed]
- Mory F, Alauzet C, Matuszeswski C, et al. Evaluation of the new Vitek 2 ANC card for identification of medically relevant anaerobic bacteria. J Clin Microbiol 2009;47:1923-6. [PubMed]
- Lee EH, Degener JE, Welling GW, et al. Evaluation of the Vitek 2 ANC card for identification of clinical isolates of anaerobic bacteria. J Clin Microbiol 2011;49:1745-9. [PubMed]
- Blairon L, Maza ML, Wybo I, et al. Vitek 2 ANC card versus BBL Crystal Anaerobe and RapID ANA II for identification of clinical anaerobic bacteria. Anaerobe 2010;16:355-61. [PubMed]
- Charnot-Katsikas A, Tesic V, Boonlayangoor S, et al. Prospective Evaluation of the VITEK® MS for the Routine Identification of Bacteria and Yeast in the Clinical Microbiology Laboratory: Assessment of Accuracy of Identification and Turnaround Time. J Med Microbiol 2014;63:235-41. [PubMed]
- Bizzini A, Greub G. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry, a revolution in clinical microbial identification. Clin Microbiol Infect 2010;16:1614-9. [PubMed]
- García P, Allende F, Legarraga P, et al. Bacterial identification based on protein mass spectrometry: A new insight at the microbiology of the 21st century. Rev Chilena Infectol 2012;29:263-72. [PubMed]
- Legarraga P, Moraga M, Lam M, et al. Impact of mass spectrometry by MALDI-TOF MS for the rapid identification of aerobic and anaerobic bacteria of clinical importance. Rev Chilena Infectol 2013;30:140-6. [PubMed]
- Biswas S, Rolain JM. Use of MALDI-TOF mass spectrometry for identification of bacteria that are difficult to culture. J Microbiol Methods 2013;92:14-24. [PubMed]
- Croxatto A, Prod’hom G, Greub G. Applications of MALDI-TOF mass spectrometry in clinical diagnostic microbiology. FEMS Microbiol Rev 2012;36:380-407. [PubMed]
- Veloo AC, Knoester M, Degener JE, et al. Comparison of two matrix-assisted laser desorption ionisation-time of flight mass spectrometry methods for the identification of clinically relevant anaerobic bacteria. Clin Microbiol Infect 2011;17:1501-6. [PubMed]
- Schmitt BH, Cunningham SA, Dailey AL, et al. Identification of anaerobic bacteria by Bruker Biotyper matrix-assisted laser desorption ionization-time of flight mass spectrometry with on-plate formic acid preparation. J Clin Microbiol 2013;51:782-6. [PubMed]
- Jamal WY, Shahin M, Rotimi VO. Comparison of two matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry methods and API 20AN for identification of clinically relevant anaerobic bacteria. J Med Microbiol 2013;62:540-4. [PubMed]
- Nagy E, Becker S, Kostrzewa M, et al. The value of MALDI-TOF MS for the identification of clinically relevant anaerobic bacteria in routine laboratories. J Med Microbiol 2012;61:1393-400. [PubMed]
- Garner O, Mochon A, Branda J, et al. Multi-centre evaluation of mass spectrometric identification of anaerobic bacteria using the VITEK® MS system. Clin Microbiol Infect 2014;20:335-9. [PubMed]
- Coltella L, Mancinelli L, Onori M, et al. Advancement in the routine identification of anaerobic bacteria by MALDI-TOF mass spectrometry. Eur J Clin Microbiol Infect Dis 2013;32:1183-92. [PubMed]
- Nagy E, Maier T, Urban E, et al. Species identification of clinical isolates of Bacteroides by matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry. Clin Microbiol Infect 2009;15:796-802. [PubMed]
- Veloo AC, Erhard M, Welker M, et al. Identification of Gram-positive anaerobic cocci by MALDI-TOF mass spectrometry. Syst Appl Microbiol 2011;34:58-62. [PubMed]
- Kierzkowska M, Majewska A, Kuthan RT, et al. A comparison of Api 20A vs MALDI-TOF MS for routine identification of clinically significant anaerobic bacterial strains to the species level. J Microbiol Methods 2013;92:209-12. [PubMed]
- Vega-Castaño S, Ferreira L, González-Ávila M, et al. Reliability of MALDI-TOF mass spectrometry in the identification of anaerobic bacteria. Enferm Infecc Microbiol Clin 2012;30:597-601. [PubMed]
- La Scola B, Fournier PE, Raoult D. Burden of emerging anaerobes in the MALDI-TOF and 16S rRNA gene sequencing era. Anaerobe 2011;17:106-12. [PubMed]
- Clarridge JE 3rd. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev 2004;17:840-62. [PubMed]
- Veloo AC, Welling GW, Degener JE. The identification of anaerobic bacteria using MALDI-TOF MS. Anaerobe 2011;17:211-2. [PubMed]
- Lau SK, Tang BS, Teng JL, et al. Matrix-assisted laser desorption ionisation time-of-flight mass spectrometry for identification of clinically significant bacteria that are difficult to identify in clinical laboratories. J Clin Pathol 2014;67:361-6. [PubMed]
- Tan KE, Ellis BC, Lee R, et al. Prospective evaluation of a matrix-assisted laser desorption ionization-time of flight mass spectrometry system in a hospital clinical microbiology laboratory for identification of bacteria and yeasts: a bench-by-bench study for assessing the impact on time to identification and cost-effectiveness. J Clin Microbiol 2012;50:3301-8. [PubMed]


