Body mass index of patients with chronic obstructive pulmonary disease is associated with pulmonary function and exacerbations: a retrospective real world research
Introduction
In China, the prevalence of chronic obstructive pulmonary disease (COPD) is high both in urban and rural area (1,2). The morbidity and mortality of COPD have a trend of increase in recent years. The previous COPD researches demonstrated that frequent exacerbations and accelerated decline of pulmonary function could lead to disease progression (3). Current evidence showed some drugs and interventions could partially delay COPD progression. So it is essential to find indicators to predict the prognosis and outcome of COPD.
As we all know, obesity played a detrimental role in asthma and obstructive sleep apnea hypopnea syndrome (4-6). However, conclusions on the roles of body mass index (BMI) in COPD were discrepant. It has been demonstrated that higher mortality in underweight COPD patients was partially due to the accelerated decline of forced expiratory volume in 1 second (FEV1) (3,7). Unfortunately, most clinical studies concentrated on the effect of low weight and severe obesity on the mortality of patients with COPD, but few studies focused on the relationship of BMI and other factors during the progression of COPD (8-12). Moreover, most of the previous studies were only small sample randomized controlled trials, which limited to generalize the conclusions. Therefore, it would be meaningful to add data from real world and study other factors. The aim of our retrospective research was to study the effects of BMI on COPD progression.
Methods
Study design and patients
With a retrospective real world research design, all cases with COPD as one of discharge diagnosis in Shandong Provincial Hospital or Shanghai Zhongshan Hospital from January 1st, 2014 to December 31st, 2016 were gathered from hospital records databases. Patients must be over 40 years old, had records of pulmonary function tests, and be alive when discharged from hospital.
Data collection
All data during the above mentioned period were sequentially collected by manual extraction from electronic medical records through hospital records databases in two centers, including basic information (gender, age, height and weight), history of smoking, co-morbidities, laboratory blood tests—white blood cell (WBC), neutrophils, eosinophils, C-reactive protein (CRP), pulmonary function tests—forced expiratory volume in 1 second (post bronchodilator FEV1), forced expiratory vital capacity (FVC), peak expiratory flow (PEF), forced expiratory flow (FEF25–75) and diffusing capacity of carbon monoxide (DLCO), pulmonary arterial pressure (PAP) measured by Doppler cardiac ultrasound, the dose of systemic corticosteroid and length of stay. From March to May of 2017, all recruited patients were followed up by phone calls and hospital record systems to record their moderate and severe acute exacerbation events from January 1st, 2014 to December 31st, 2016.
Study variables
BMI was used as the independent variable. According to the standard of the World Health Organization (WHO), all participants were classified into four subgroups, underweight (BMI <18.5), normal weight (18.5≤ BMI <25), overweight (25≤ BMI <30) and obesity (BMI ≥30).
Parameters in pulmonary function tests and frequency of exacerbation were used as observable variables. (I) post bronchodilator FEV1%pred (here after termed FEV1)was divided into four degrees according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2014: GOLD1 (FEV1 ≥80%pred), GOLD2 (50%pred ≤ FEV1 <80%pred), GOLD3 (30%pred≤ FEV1 <50%pred), GOLD4 (FEV1 <30%pred). It is widely used to predict the severity of COPD (3). (II) PEF is defined as the maximal flow (or speed). To some degree, it could reflect the condition of airway obstruction, especially when combined with FEF25 to indicate the status of large airways and respiratory muscles strength. (III) FEF is usually presented as intervals of FEF25, FEF50 and FEF75, which represent FEF after 25%/50%/75% of FVC has been exhaled respectively. FEF25 is an indicator to reflect the flow of the early stage of expiration and will decline if large airway is obstructive. FEF50 and FFE75 reflect the flow of the middle stage and the later stage of expiration. FEF25–75 or FEF50–75 may be more sensitive parameters than FEV1 in the assessment of small airway function. (IV) DLCO is used to reflect pulmonary diffusion function. (V) total times of exacerbations is the sum of moderate and severe exacerbations in three years. The definitions of acute exacerbation and its severities are according to GOLD 2014.
Statistical analysis
Data collection and record used EpiData (Version 3.1). Statistical analysis was performed using SPSS Version 23.0 (SPSS Inc.; Chicago, Illinois) and GraphPad Prism 7 (GraphPad, San Diego, CA). Parametric data were presented as mean ± standard error of the mean (SEM) or range. Non-parametric data were presented as median (interquartile range, IQR). We used Spearman bivariate correlations to investigate the correlation of BMI to each indicator. In the primary analysis, Kruskal-Wallis test was used to compare difference among multi-groups and then Mann-Whitney U test was used to compare in pairs. In Kruskal-Wallis test, variation tendency was analyzed by Mean Rank. The chi-squared test (χ2) was used to contrast proportions among groups. In the secondary analysis, the ordinal multinomial logistic regression was used to analyze multivariable parameters. P<0.05 was considered as the threshold of significance for all statistical analysis.
Results
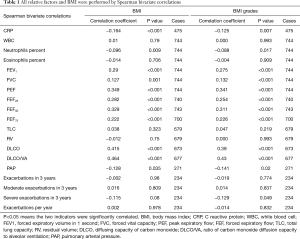
Finally, 744 COPD patients were retained (Figure 1). BMI had mild or moderate positively correlation with FEV1, PEF, FEF25–75 and DLCO (Table 1).
Full table
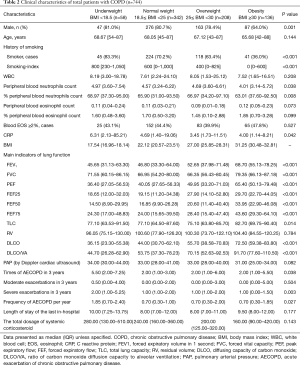
In 744 cases, 77% were male with a median age (IQR) of 67 years. Among them, 57.53% were smokers and 32.93% were non-smokers. The smoking-history of other 9.54% patients was unclear. According to BMI grades, all cases were classified into four groups as follows: underweight (58 cases, BMI 17.54), normal weight (342 cases, BMI 22.12), overweight (208 cases, BMI 27.00) and obesity (136 cases, BMI 31.25) (Table 2).
Full table
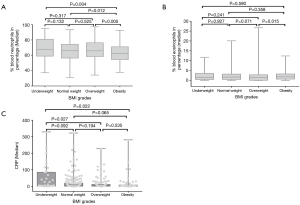
There was no significant difference of WBC count among groups. The percentage of neutrophils presented significant declining trend with the increasing of BMI. As compared to obesity group, the value in each other three groups was significantly higher. But there was no marked difference among underweight, normal weight and overweight groups (Figure 2A). The percentage of eosinophils in obesity groups was higher than that of underweight, normal weight and overweight groups. The difference was only significant as compared to that of overweight group (Figure 2B). The value of CRP presented declining trend with the increasing of BMI. It was highest in underweight group, which was significantly higher than those of overweight and obesity groups but not that of normal weight group (Figure 2C).
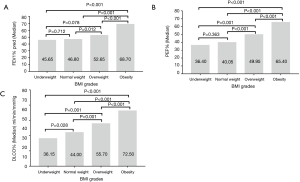
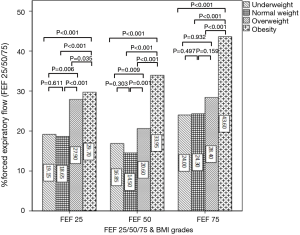
FEV1, PEF and DLCO were all significantly increased with the increase of BMI, except for the not significant differences between underweight and normal weight groups in FEV1 and PEF (Figure 3A,B,C). The overall trend of FEF25, FEF50 and FEF75 presented increasing with the increase of BMI (Figure 4). The values of FEF25, FEF50 and FEF75 in underweight and normal weight groups were similar, which were significantly lower than those of overweight and obesity groups except for FEF75. All FEF25, FEF50 and FEF75 in obesity group were significantly higher than those of overweight group.
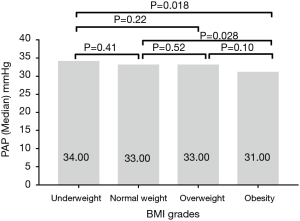
All together 271 patients had the Doppler cardiac ultrasound examination. Only the PAP in underweight group was higher than 40 mmHg. The PAP value was conversely correlated to BMI. It was markedly lower in obesity population than that of underweight or normal weight groups but not significantly among other groups comparison (Figure 5).
The length of stay and total dosage of systemic corticosteroid were not notably different among four BMI groups (Table 2).
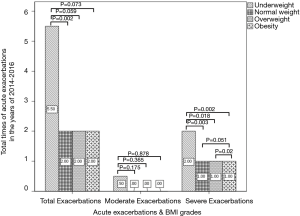
The frequency of total exacerbations and severe exacerbations per year were significantly higher in underweight group than those in other three groups (Figure 6).
Subgroups analysis
Due to the baseline, such as gender and smoking, was not matched. We analyzed those indicators in each subgroup—male, female, smokers, non-smokers in order to keep the comparability among each BMI grades.
In each subgroup, the baseline is equally comparable. The results showed FEV1, PEF, DLCO, FEF25, FEF50 were all presented significant difference according to BMI deviation in male subgroup and in female subgroup (Table 3). The tendency of FEF75 in male patients was similar to that of whole samples (Table 4). Interestingly, the total lung capacity (TLC) and residual volume (RV) in female patients presented significant increasing trend with the increase of BMI but not in male patients. Like the whole sample, the frequency of severe exacerbation per year in underweight group was much higher than those of other three groups in male patients but not in female patients. FEV1, PEF, FEF25–75 and DLCO were significantly lower in underweight group compared to obesity group both in smokers’ subgroup (Table 5) and non-smokers subgroup (Table 6). Compared with other three groups, the frequency of exacerbation and severe exacerbation per year of underweight patients was significantly higher in smoking patients. Those results manifested the conclusion of BMI and lung function and exacerbation is available.
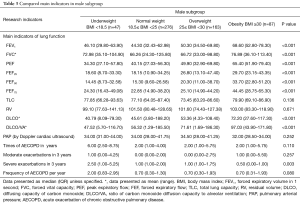
Full table
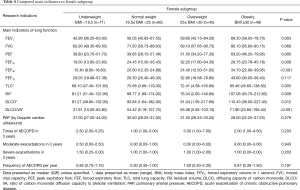
Full table
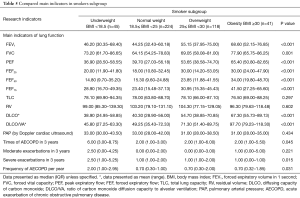
Full table
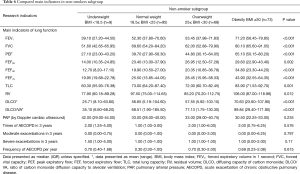
Full table
Secondary analysis
FEV1 is the most important indicator for COPD patients in lung function; GOLD grade is an indicator which could be standing for FEV1 level in COPD. We performed ordinal multinomial logistic regression and choose GOLD grades as the dependent variable; age, gender, BMI, smoking status, WBC, neutrophils and eosinophils %, CRP and PAP as independent variables. Age, gender, BMI, PAP presented statistical significance. According to the results of optimization model, the Exp value was calculated. Compared with elder COPD patients, the youngers were possibly in better GOLD stages (Exp=1.052). Compared with female COPD patients, the males were possibly in worse GOLD stages (Exp=2.149). Compared with obesity class respectively, the underweight and normal weight patients were possibly in worse GOLD stages (Exp=3.297 and Exp=2.821). Compared with patients with PAP >30 mmHg (13), the patients with PAP ≤30 mmHg were possibly in better GOLD stages (Exp=0.571) (Table 7).
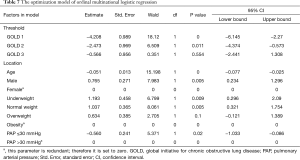
Full table
Discussion
The effect of BMI in the progression of COPD drew lots of attention (14). Our retrospectively real world research demonstrated that in patients with COPD, BMI was positively correlated with pulmonary function and negatively correlated with inflammation levels and acute exacerbations markedly.
It is generally accepted that improving nutrition status, enhancing respiratory muscles strength and reducing inflammation level are effective on long-term management of COPD (15). BMI was considered as an accurate indicator of nutrition in some degree. The prevalence of sarcopenia was common in COPD patients, especially in severe, elder or underweight patients (16). As shown in our results, compared to patients with COPD in lower BMI, those in higher BMI (overweight and obesity) had better pulmonary function, lower inflammation level (lower CRP and neutrophils) and less exacerbation. Better pulmonary function in our study were higher FEV1%pred to show slighter airway obstruction, higher PEF and FEF25 or FEF50 and FEF75 to indicate better major or small airways function, and higher DLCO to present better lung diffusion function. In our study, no matter in total samples or subgroups divided by gender, smoking status, acute or stable COPD, the results were all consistent. All above results indicated that the overall prognosis might be better with higher BMI. We hypothesized the reasons might be: firstly, even though we did not analyze COPD patients in “blue bloater” or “pink puffer” clinical phenotypes, generally speaking, overweight and obesity COPD patients might have more nutrition intake and be in better nutrition status than underweight patients; secondly, bigger in size might let the patients have more and stronger muscles to facilitate breathing. Then, they could do more exercise and had more and stronger muscles in turn; thirdly, lower inflammation level could reduce the possibility of acute exacerbations (15,17-22). Therefore, BMI might be an important indicator to evaluate COPD patients’ condition and long-term chronic diseases management.
Due to innate idea, some doctors think that obesity people may have glucocorticoid resistance (23), which seems that patients with COPD in obesity might need higher systemic corticosteroid doses and longer treatment for acute exacerbations. On the contrary, our research showed decreasing tendency in dose of systemic corticosteroid and length of stay with increasing of BMI grades, which also demonstrated that higher BMI might be a protective factor.
We further analyzed the effect of smoking. Different from non-smoking patients, acute moderate-severe exacerbations happened significantly less with the increase of BMI grades in smoking subgroups. In male COPD patients, the smokers were the majority and the exacerbations was significantly less with the increase of BMI. So, it would be important for male, smoking, underweight COPD patients to gain higher BMI in order to improve their prognosis. Interestingly, Schermer et al. also recently supported our results and indicated that higher BMI might be beneficial to ameliorate airway obstruction (24).
In our study, most of patients with COPD in obesity were in obesity class I (BMI 30–34.9 kg/m2) (25), which were similar to the BMI distribution in total Chinese population (26). We found patients with COPD in BMI≥25 (overweight and obesity class I) had better pulmonary function, less exacerbations, lower PAP and lower inflammation level, compared with patients with COPD in underweight. Our results are partially opposite to some previous studies. Lambert’s research showed patients with COPD in obesity were prevalent, and the increase of BMI was related to co-morbidities increasing, life quality declining, pulmonary function damaging, severe exacerbation risk rising and worse prognosis (10). Chilean researchers held the idea that CRP was weakly associated with fat mass/BMI and frequency of exacerbations (27). Weinreich et al. and Pekkarinen et al. considered lean body mass positively correlated with DLCO (28,29). The reason for this phenomenon is possibly that COPD patients in obesity in our study were all in lower obesity grade (BMI 30–34.9 kg/m2), compared with COPD patients from western countries (nearly half of obesity patients were in BMI ≥35 kg/m2), this phenomenon is more consistent with the characteristics of Asians (10,25,26,30,31). Notably, in recent years, occidental researchers got the “Obesity paradox” conclusion in COPD domain. A research by Paul Stoll with 75 COPD patients showed that overweight and obesity were positive predictors of long-term survival in COPD patients (2,11).
For COPD patients in China, treatment is not standard; medication adherence is poor; and morbidity of COPD is still high (1,2). From our results, the healthy and properly body weight is good for long term management of COPD patients. This will be suitable for current Chinese condition, even Asian. The patients of this study were from Shandong Province Hospital and Shanghai Zhongshan Hospital. Their economic status, education levels and healthcare situation were similar to others from eastern China. So the conclusions of this study are representative. As we all know, obesity is a double-edged sword. In future studies, we will try to provide an optimal BMI cutoff point for patients with COPD to obtain benefits of better pulmonary function, lower level of inflammation and fewer exacerbations. There is a limitation that patients were followed-up three years frequency of AECOPD may have recall-bias in this retrospective research. Another, BMI may be not a good indicator to reflex the status of nutrition and muscles. So in next perspective study, we will pay attention to the role of body composition and fat free mass index in COPD patients.
Conclusions
In our study, with the increase of BMI, the pulmonary function improved while the inflammation level and frequencies of exacerbations decreased. So, BMI might be a useful indicator to predict the prognosis of patients with COPD. This study provided a good idea for further related research and COPD long-term management.
Acknowledgements
We should give our thanks to the medical record department of Shandong Provincial Hospital to offer those COPD patients’ hospitalization records.
Funding: This work was partially supported by Natural Science Foundation of China (Project number: 81300030, 81570336).
Footnote
Conflicts of Interest: The authors report no conflicts of interest in this work.
Ethical Statement: As there was a retrospective study, no randomization, no new treatment being explored and no potential harm to the patients. This study was approved by Shandong Provincial Hospital Medical Ethics Committee (Ethical Review of Medical Research on Human Being No. 2016-23).
References
- Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med 2007;176:753-60. [Crossref] [PubMed]
- Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet 2018;391:1706-17. [Crossref] [PubMed]
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). Available online: http://goldcopd.org. 2014.
- Peters U, Dixon AE. Obesity and asthma. J Allergy Clin Immunol 2018;141:1169-79. [Crossref] [PubMed]
- Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med Rev 2017;34:70-81. [Crossref] [PubMed]
- Mathew JL, Narang I. Sleeping too close together: obesity and obstructive sleep apnea in childhood and adolescence. Paediatr Respir Rev 2014;15:211-8. [Crossref] [PubMed]
- Kivimäki M, Shipley MJ, Bell JA, et al. Underweight as a risk factor for respiratory death in the Whitehall cohort study: exploring reverse causality using a 45-year follow-up. Thorax 2016;71:84-5. [Crossref] [PubMed]
- Guo Y, Zhang T, Wang Z, et al. Body mass index and mortality in chronic obstructive pulmonary disease: A dose-response meta-analysis. Medicine (Baltimore) 2016;95. [Crossref] [PubMed]
- Eriksson B, Backman H, Bossios A, et al. Only severe COPD is associated with being underweight: results from a population survey. ERJ Open Res 2016;2:00051-2015. [Crossref] [PubMed]
- Lambert AA, Putcha N, Drummond MB, et al. Obesity Is Associated With Increased Morbidity in Moderate to Severe COPD. Chest 2017;151:68-77. [Crossref] [PubMed]
- Stoll P, Foerster S, Virchow JC. Overweight is a predictor of long-term survival in hospitalized patients with exacerbations of COPD. Respir Med 2016;116:59-62. [Crossref] [PubMed]
- Jordan JG, Mann JR. Obesity and mortality in persons with obstructive lung disease using data from the NHANES III. South Med J 2010;103:323-30. [Crossref] [PubMed]
- Zhang RF, Zhou L, Ma GF, et al. Diagnostic value of transthoracic Doppler echocardiography in pulmonary hypertension: a meta-analysis. Am J Hypertens 2010;23:1261-4. [Crossref] [PubMed]
- Prescott E, Almdal T, Mikkelsen KL, et al. Prognostic value of weight change in chronic obstructive pulmonary disease: results from the Copenhagen City Heart Study. Eur Respir J 2002;20:539-44. [Crossref] [PubMed]
- Vanfleteren LEGW, Spruit MA, Wouters EFM. Management of chronic obstructive pulmonary disease beyond the lungs. Lancet Respir Med 2016;4:911-24. [Crossref] [PubMed]
- Jones SE, Maddocks M, Kon SS, et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax 2015;70:213-8. [Crossref] [PubMed]
- Hallin R, Koivisto-Hursti UK, Lindberg E. Nutritional status, dietary energy intake and the risk of exacerbations in patients with chronic obstructive pulmonary disease (COPD). Respir Med 2006;100:561-7. [Crossref] [PubMed]
- Skyba P, Kluchova Z, Joppa P, et al. Nutritional status in relation to respiratory impairment and systemic inflammation in patients with acute exacerbations of COPD. Med Sci Monit 2009;15:CR528-33. [PubMed]
- Landbo C, Prescott E, Lange P, et al. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:1856-61. [Crossref] [PubMed]
- Ora J, Laveneziana P, Wadell K, et al. Effect of obesity on respiratory mechanics during rest and exercise in COPD. J Appl Physiol (1985) 2011;111:10-9. [PubMed]
- Sexton P, Metcalf P, Kolbe J. Respiratory effects of insulin sensitisation with metformin: a prospective observational study. COPD 2014;11:133-42. [Crossref] [PubMed]
- Jeon YK, Shin MJ, Kim WJ, et al. The relationship between pulmonary function and bone mineral density in healthy nonsmoking women: the Korean National Health and Nutrition Examination Survey (KNHANES) 2010. Osteoporos Int 2014;25:1571-6. [Crossref] [PubMed]
- Anderson WJ, Lipworth BJ. Does body mass index influence responsiveness to inhaled corticosteroids in persistent asthma? Ann Allergy Asthma Immunol 2012;108:237-42. [Crossref] [PubMed]
- Schermer TR, Robberts B, Crockett AJ, et al. Should the diagnosis of COPD be based on a single spirometry test? NPJ Prim Care Respir Med 2016;26:16059. [Crossref] [PubMed]
- Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med 1998;158:1855-67. [Crossref] [PubMed]
- Yang L, Zhou M, Smith M, et al. Body mass index and chronic obstructive pulmonary disease-related mortality: a nationally representative prospective study of 220,000 men in China. Int J Epidemiol 2010;39:1027-36. [Crossref] [PubMed]
- Díaz O, Parada A, Ramos C, et al. C-Reactive protein levels in patients with chronic obstructive pulmonary disease. Revista Médica De Chile 2012;140:569-78. [PubMed]
- Weinreich UM, Thomsen LP, Brock C, et al. Diffusion capacity of the lung for carbon monoxide - A potential marker of impaired gas exchange or of systemic deconditioning in chronic obstructive lung disease? Chron Respir Dis 2015;12:357-64. [Crossref] [PubMed]
- Pekkarinen E, Vanninen E, Länsimies E, et al. Relation between body composition, abdominal obesity, and lung function. Clin Physiol Funct Imaging 2012;32:83-8. [Crossref] [PubMed]
- Lim JU, Lee JH, Kim JS, et al. Comparison of World Health Organization and Asia-Pacific body mass index classifications in COPD patients. Int J Chron Obstruct Pulmon Dis 2017;12:2465-75. [Crossref] [PubMed]
- Yamauchi Y, Hasegawa W, Yasunaga H, et al. Paradoxical association between body mass index and in-hospital mortality in elderly patients with chronic obstructive pulmonary disease in Japan. Int J Chron Obstruct Pulmon Dis 2014;9:1337-46. [Crossref] [PubMed]


