Contemporary outcomes of surgical management of complex thoracic infections
Introduction
Complex thoracic infections (CTIs) frequently present in the form of complicated pneumonia, loculated pleural effusion, empyema or pulmonary abscess. Effusions frequently complicate pneumonias as migration of neutrophils and monocytes result in intrapleural inflammation and accumulation of fluid (1). Most effusions are sterile or simple, and they typically resolve as the infectious process is treated. However, when bacteria translocate into the pleural space, a complex effusion or empyema may develop. Complex effusions are characterized by the formation of fibrin deposits, septations and/or purulent deposits in the pleural space. This exudative process usually necessitates invasive drainage procedures (DP), intrapleural enzyme administration, and/or surgical washout with or without decortication to achieve resolution (1-3).
In a recent study, the majority of empyemas were found to be secondary to infections with viridans group streptococci, which was isolated in 64% of cases with cultures (3). Along with more virulent strains of common bacterial pathogens, the prevalence of non-tuberculous myobacterial lung disease has been increasing (4). The indolent nature of these complex infections, as well as high antibiotic resistance may result in extensive parenchymal destruction causing respiratory failure and recurrent infections. Traditional teaching has cautioned against the use of parenchymal resections for the management of complex pulmonary infections, with a reported mortality of up to 40%. More recently, the American Thoracic Society has proposed a multidisciplinary treatment approach for complicated infectious lung diseases, which involves a combination of antibiotics and adjuvant surgery (4,5).
The objectives of this study were to evaluate the contemporary use of surgical management for CTIs and compare 30-day morbidity and mortality of thoracic DP to those that involved lung resection (LR) using a multi-institutional validated, standardized, and audited surgical outcomes database.
Methods
The American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) is a prospective, multi-institutional, risk-adjusted 30-day outcomes program that provides participating hospitals with comparative data for internal quality-improvement efforts. The details of the sampling strategy, data abstraction, variables collected, and outcomes monitored have been previously described (6). Institutional review board approval was not obtained for this study given the lack of patient identifying data in the ACS NSQIP database.
The ACS NSQIP participant use file (PUF) from 2014 to 2017 was used to identify patients who underwent a thoracic surgical intervention including: bilobectomy, decortication, exploration of the chest with biopsy, partial removal of the lung, release of lung, removal of lung/lobectomy, thoracoscopic lobectomy, thoracoscopic pleurodesis, thoracoscopic wedge resection, thoracoscopic biopsy of the pleura, thoracoscopy, thoracotomy with major exploration, thoracotomy with wedge resection, thoracotomy with decortication, and thoracotomy with lobectomy based on Current Procedural Terminology (CPT codes: 32100, 32220, 32480, 32482, 32505, 32609, 32650, 32652, 32663, 32666). Patients with CTIs were selected based on ICD-9 codes for empyema, pneumonia, pulmonary abscess, aspergillosis, tuberculosis, coccidiomycosis, histoplasmosis, pneumocystosis, candida, actinomycosis, cryptococcosis, viral pneumonia, mycobacteria, and zygomycosis. Patients were then classified into two groups based on extent of surgery: DP and LR.
To reduce confounding from multiple procedures, patients who had undergone a prior operation within 30 days of the index procedure were excluded from the analysis. Patient demographics, baseline comorbidities and clinical characteristics were recorded (6). Additional, procedure specific variables including transfusion requirement, operative time and total length of stay were assessed, as was surgeon specialty (i.e., thoracic, cardiac, or general). Surgical extent was based on the most invasive CPT code listed.
The primary outcome was overall 30-day postoperative mortality. Secondary outcomes included overall morbidity rate, specific complications rates, need for reoperation within 30 days of the index operation, postoperative length of stay, discharge status, as well as standard postoperative complication rates (6). Descriptive statistics and a univariate analysis were executed using student t-test and Chi-squared, as appropriate. Multivariate analysis was executed using logistic regression. A P value <0.05 was considered statistically significant with a confidence interval of 95%.
Results
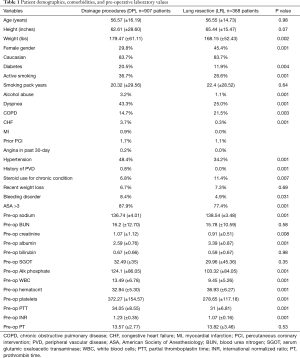
A total of 4,205 patients were identified between 2014 and 2017. Surgical management of a CTI was performed in 1,275 patients (30.3%). The mean age was 56.6±15.8 years, 83.7% were Caucasian, and 34.3% female. A LR (partial or anatomical) was performed in 28.9% (368/1,275) of patients operated on for CTI. Patient demographics, comorbidities, and pre-operative laboratory values are presented in Table 1. There was no difference in mean age (56.57±16.19 vs. 56.55±14.73, P=0.98), or race (Caucasian 83.7% for both, P=0.1) in the DP and LR groups, respectively. However, female gender was significantly higher in the DP group than in the LR group (29.8% vs. 45.4%, P=0.001); and diabetes was significantly lower (20.5% vs. 11.9%, P=0.004). Patients in the DP group were more likely to be active smokers (36.7% vs. 26.6%, P=0.001), have a history of alcohol abuse (3.2% vs. 1.1%, P=0.001), dyspnea on exertion (43.3% vs. 25.0%, P=0.001), congestive heart failure (3.7% vs. 0.3%, P=0.001), hypertension (48.4% vs. 34.2%, P=0.001), and American Society of Anesthesiologist (ASA) classification >3 (87.9% vs. 77.4%, P=0.001), compared to patients in the LR group. Conversely, patients in the LR group were more likely to have COPD (14.7% vs. 21.5%, P=0.003) and be on chronic steroids (6.8% vs. 11.4%, P=0.007). There was no difference in smoking pack years or recent weight loss between the two groups. Preoperative laboratory values are also listed in Table 1.
Full table
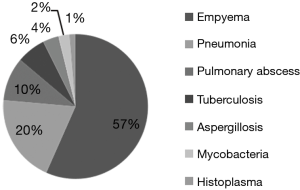
Overall, the indications for surgery were empyema 57% (n=667), pneumonia 20% (n=223), pulmonary abscess 10% (n=115), aspergillosis 4% (n=42), tuberculosis 6% (n=76), coccidiomycosis 0.9% (n=11), histoplasmosis 1.3% (n=16), pneumocystosis 0.2% (n=3), candida 0.1% (n=1), actinomycosis 0.3% (n=4), cryptococcosis 0.6% (n=7), viral pneumonia 0.2% (n=3), mycobacteria 2% (n=29), and zygomycosis 0.4% (n=5) (Figure 1).
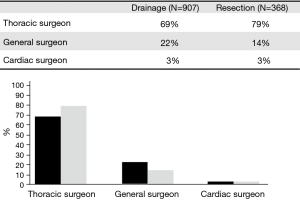
The operative surgeon was identified as a Thoracic surgeon in 69%, General surgeon in 22%, and Cardiac surgeon in 3% of cases. In general, Thoracic surgeons were more likely to perform a LR than General or Cardiac Surgeons (79% vs. 14% and 3%, respectively, P=0.001). Figure 2 represents the credentials of the operating surgeon in the DP and LR groups. The most common procedure was a total decortication, which was performed in 43% (n=549) of cases. Other cases included: thoracoscopy with washout 11% (n=136), thoracoscopic wedge resection 8% (n=105), release of lung 8% (n=98), single lobectomy 7% (n=97), thoracotomy with wedge resection 5% (n=59), thoracoscopic lobectomy 4% (n=50), thoracic exploration with biopsy 3% (n=38), partial removal of the lung 3% (n=35), thoracoscopic pleurodesis 3% (n=34), thoracoscopic pleural biopsy 3% (n=32), thoracotomy 3% (n=32), and bilobectomy 1% (n=10).
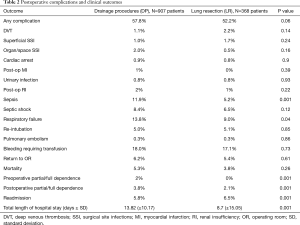
Overall, a postoperative complication occurred in 56.2% of cases (716/1,275). Postoperative outcomes are listed in Table 2. On univariate analysis, there was no significant difference in the rate of any complication between the DP and LR groups (57.8% vs. 52.2%, P=0.06). However, the DP group experienced significantly higher frequency of respiratory failure (13.8% vs. 9.0%, P=0.04) and sepsis (11.9% vs. 5.2%, P=0.001) compared to the LR group. There was no difference in the rates of re-intubation (5.0% vs. 5.1%, P=0.85), surgical site infections (1.0% vs. 1.7%, P=0.24), urinary tract infections (0.8% vs. 0.8%, P=0.93), return to the operating room (6.2% vs. 5.4%, P=0.61), cardiac arrest (0.9% vs. 0.8%, P=0.9), deep venous thrombosis (1.1% vs. 2.2%, P=0.14), septic shock (8.4% vs. 6.5%, P=0.12), transfusion requirement (18.0% vs. 17.1%, P=0.73), renal failure (2% vs. 1%, P=0.22), and pulmonary embolism (0.3% vs. 0.3%, P=0.86) between the two groups. The mean total length of hospital days was longer in the DP group compared to the LR group (13.8±10.17 vs. 8.7±15.05, P=0.001), and patients in the DP group were more likely to be discharged to a rehabilitation center (3.8% vs. 2.1%, P=0.001). Patients in the LR group did have a slightly higher rate of readmission within 30-days (5.8% vs. 6.5%, P=0.001). There was no significant difference in 30-day mortality between the DP and the LR group (5.3% vs. 3.8%, P=0.26).
Full table
On multivariate analysis, 30-day mortality was associated with age [odds ratio (OR) 1.04; 95% CI, 1.02–1.07], preoperative steroid use (OR 2.76; 95% CI, 1.02–7.57), renal failure (OR 1.55; 95% CI, 1.23–1.97), leukocytosis (OR 1.07; 95% CI, 1.02–1.12), pulmonary embolism (OR 34.11; 95% CI, 2.42–480.88), sepsis (OR 7.04; 95% CI, 1.27–38.46), and any postoperative complication (OR 4.23; 95% CI, 1.46–12.24). The regression model had a concordance statistic of 0.87.
Discussion
New evidence is beginning to emerge that challenges traditional surgical dogma of avoiding LR for CTIs. In the current study, we describe the contemporary, real world outcomes of 1,275 patients across the United States who underwent surgical management of a CTI during 2014 to 2017 in ACS-NSQIP participating hospitals. A LR procedure was performed in 28.9% of identified patients with ICD-9 codes indicating an infectious etiology. The patients identified were predominantly male, Caucasian and relatively young, with a mean age in the mid-50s. While there were some baseline differences in the co-morbidities of the two groups, it appears from our data that cases of CTI that require a parenchymal resection can be done with similar rates of overall complications, no difference in 30-day mortality, and lower length of stay compared to those cases that only required pleural drainage.
While some type of pleural intervention is generally thought to be required for empyema treatment, a recent study by Birkenkamp et al. described the outcomes of 91 patients who underwent conservative management for empyema (3). The predominant organisms were viridans group streptococci in 64% of cases with a median length of hospitalization of 9 days and the duration of antimicrobial therapy ranged between 15 and 31 days. The use of longer courses of parenteral antibiotic therapy was associated with fewer cases of clinical failure. However, adjunct therapies, such as drainage or surgical washout, utilized for the management of these infections were not mentioned in the study.
Intrapleural tissue plasminogen activator (tPA)/deoxyribonuclease (DNase) therapy for pleural infection has been shown to significantly improve radiological outcomes in several studies (7-14). In a multicenter study reported by Piccolo et al., 107 patients with pleural infections were treated with antibiotics and drainage (7). The majority of patients (92.3%) were successfully managed without the need for surgical intervention. Most patients (84%) received tPA/DNase more than 24 hours after failing to respond to initial conservative management with antibiotics and tube thoracostomy. The administration of tPA/DNase increased fluid drained by a factor of 10 over a period of 72 hours after the initial administration. The investigators noted a corresponding clearance of pleural opacity on chest radiographs as well as significant reduction in C-reactive protein after therapy. Interestingly, in regards to surgical management, a systematic review published by Redden et al. concluded that there is no statistically significant difference in mortality between primary surgical and non-surgical management of pleural empyema for all age groups (15). However, video-assisted thoracoscopic surgery reduced length of hospital stay compared to tube thoracostomy drainage alone.
The most frequent indications for surgical intervention in the current study were empyema (57%), complicated pneumonia (20%), pulmonary abscess (10%), and tuberculosis (6%). The goal of surgery in the management of complicated infections is to disrupt undrained or trapped purulent collections to either free or prevent the formation of trapped lung and allow the infection to clear. Traditionally, indications for resection of lung parenchyma in cases of infection include a poor response to drug therapy, the development of drug resistant disease, or the presence of a significant disease related complication such as hemoptysis (4). The surgical procedures of choice are various types of pulmonary resections, including wedge resection, segmentectomy, lobectomy, or pneumonectomy.
The outcomes of 171 patients who underwent anatomic LR with thoracoscopic lobectomy or segmentectomy for infectious lung disease were reported by Mitchell et al. in 2012 (16). Eighty six percent of these patients had a Mycobacterium avium complex infection. The authors recommend the use of video assisted thoracoscopy as the main approach for the management of lung infections, mainly due to the potentially lower risk of developing a bronchopleural fistula. The mortality rate that had been previously reported was 7.1% in the 1980s, 4.8% in the 1990s, and 0.6% in the 2000s. In the current study, the 30-day mortality for patients in the DP group was 5.3%, and 3.8% in the LR group (P=0.26), indicating resection can be done without increasing mortality. Our multivariate analysis revealed that an increased risk of 30-day mortality was associated with age, preoperative steroid use, renal failure, leukocytosis, pulmonary embolism, and sepsis with a concordance score showing high predictability (0.87). These results seem in-line with other contemporary series (4,16-20). Taken together, these data suggest that the appropriate use of LR for infectious lung disease can be safe and beneficial.
The findings of this study should be interpreted in the setting of several limitations. Given the retrospective nature of the data, comparisons of these treatment groups can suffer from selection bias in the treatment assignment. It is also difficult to determine if a LR was planned or if this was a clinical decision made during the operation. Nevertheless, we are able to compare the extent of surgery ultimately received by the patient, which affords a more realistic assessment of the potential complications. There is also the potential underestimation of important outcome variables. However, the ACS-NSQIP database has trained, audited data abstractors and is considered by many to be the most robust large-scale surgical outcomes database available. An additional limitation of the ACS-NSQIP database is the inability to track thoracic-specific outcomes that are available in other databases, such as duration of air leak or chest tube duration. Despite these limitations, we believe that this study has the strengths of a rigorously reported, national sampling of contemporary outcomes of surgical management of CTIs and yields strong, generalizable results.
Conclusions
CTI’s are a common indication for thoracic surgical management. This contemporary, national sampling demonstrates that approximately one third of identified cases were associated with a LR. These cases demonstrated a comparable morbidity and mortality with surgical DP, but shorter hospital stays. To aid in the management of these complex disease processes, early consultation of multidisciplinary management service for these patients should be considered. Furthermore, the appropriate use of LR for infectious etiologies may lead to safer postoperative outcomes than previously thought.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Institutional review board approval was not obtained for this study given the lack of patient identifying data in the ACS NSQIP database.
References
- Koegelenberg CF, Diacon AH, Bolliger CT. Parapneumonic pleural effusion and empyema. Respiration 2008;75:241-50. [Crossref] [PubMed]
- Wrightson JM, Davies RJ. The approach to the patient with a parapneumonic effusion. Semin Respir Crit Care Med 2010;31:706-15. [Crossref] [PubMed]
- Birkenkamp K, O'Horo JC, Kashyap R, et al. Empyema management: A cohort study evaluating antimicrobial therapy. J Infect 2016;72:537-43. [Crossref] [PubMed]
- Shiraishi Y. Surgical treatment of nontuberculous mycobacterial lung disease. Gen Thorac Cardiovasc Surg 2014;62:475-80. [Crossref] [PubMed]
- Morimoto K, Iwai K, Uchimura K, et al. A steady increase in nontuberculous mycobacteriosis mortality and estimated prevalence in Japan. Ann Am Thorac Soc 2014;11:1-8. [Crossref] [PubMed]
- American College of Surgeons. Userguide for the 2009 participant use data file [homepage on the Internet; updated 2010]. Available online: http://site.acsnsqip.org/participantuse-data-file/
- Piccolo F, Pitman N, Bhatnagar R, et al. Intrapleural tissue plasminogen activator and deoxyribonuclease for pleural infection. An effective and safe alternative to surgery. Ann Am Thorac Soc 2014;11:1419-25. [Crossref] [PubMed]
- Bouros D, Schiza S, Patsourakis G, et al. Intrapleural streptokinase versus urokinase in the treatment of complicated parapneumonic effusions: a prospective, double-blind study. Am J Respir Crit Care Med 1997;155:291-5. [Crossref] [PubMed]
- Bouros D, Schiza S, Tzanakis N, et al. Intrapleural urokinase versus normal saline in the treatment of complicated parapneumonic effusions and empyema. A randomized, double-blind study. Am J Respir Crit Care Med 1999;159:37-42. [Crossref] [PubMed]
- Thommi G, Shehan JC, Robison KL, et al. A double blind randomized cross over trial comparing rate of decortication and efficacy of intrapleural instillation of alteplase vs placebo in patients with empyemas and complicated parapneumonic effusions. Respir Med 2012;106:716-23. [Crossref] [PubMed]
- Davies RJ, Traill ZC, Gleeson FV. Randomised controlled trial of intrapleural streptokinase in community acquired pleural infection. Thorax 1997;52:416-21. [Crossref] [PubMed]
- Diacon AH, Theron J, Schuurmans MM, et al. Intrapleural streptokinase for empyema and complicated parapneumonic effusions. Am J Respir Crit Care Med 2004;170:49-53. [Crossref] [PubMed]
- Moulton JS, Benkert RE, Weisiger KH, et al. Treatment of complicated pleural fluid collections with image-guided drainage and intracavitary urokinase. Chest 1995;108:1252-9. [Crossref] [PubMed]
- Maskell NA, Davies CW, Nunn AJ, et al. U.K. Controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005;352:865-74. [Crossref] [PubMed]
- Redden MD, Chin TY, van Driel ML. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst Rev 2017;3. [PubMed]
- Mitchell JD, Yu JA, Bishop A, et al. Thoracoscopic Lobectomy and Segmentectomy for Infectious Lung Disease. Ann Thorac Surg 2012;93:1033-9; discussion 1039-40. [Crossref] [PubMed]
- van Ingen J, Verhagen AF, Dekhuijzen PN, et al. Surgical treatment of nontuberculous mycobacterial lung disease: strike in time. Int J Tuberc Lung Dis 2010;14:99-105. [PubMed]
- Shiraishi Y, Katsuragi N, Kita H, et al. Adjuvant surgical treatment of nontuberculous mycobacterial lung disease. Ann Thorac Surg 2013;96:287-91. [Crossref] [PubMed]
- Yamada K, Sugiyama T, Yasuda A, et al. A study of relapse/recurrence cases after surgical treatment for patients with pulmonary nontuberculous mycobacteriosis. Kekkaku 2013;88:469-75. [PubMed]
- Subotic D, Yablonskiy P, Sulis G, et al. Surgery and pleuro-pulmonary tuberculosis: a scientific literature review. J Thorac Dis 2016;8:E474-85. [Crossref] [PubMed]