Serum decorin is a potential prognostic biomarker in patients with acute exacerbation of idiopathic pulmonary fibrosis
Introduction
Acute exacerbation (AE) of idiopathic pulmonary fibrosis (IPF) is an acute clinically significant respiratory deterioration without an obvious clinical cause (1). Three cases of AE-IPF was first reported by Kondoh et al. (1), and AE has been reported to occur at frequencies of 8.6% and 23.9% at 1 and 3 years after diagnosis of IPF, respectively (2). The mortality rate after AE onset is approximately 50% (3). Although AE had been reported in IPF patients, growing evidence has shown that AE can occur in patients with other types of fibrosing lung diseases, such as idiopathic nonspecific interstitial pneumonia and connective tissue disease-associated interstitial pneumonia (4-6). AE is the critical event in most patients with several types of interstitial pneumonia. However, the pathogenesis of AE is poorly understood and no standard treatment is yet available.
Decorin is a small leucine-rich repeat proteoglycan with one chondroitin/dermatan sulfate glycosaminoglycan side chain (7). It plays a critical role in collagen fibrillogenesis by binding to collagen, and is reported to inhibit functions of transforming growth factor (TGF)-β, a well-known profibrotic growth factor (8-10). In IPF, decorin is expressed in fibrotic lesions such as fibroblastic foci (11); additionally, it has been demonstrated that intratracheal instillation of recombinant decorin and decorin overexpression in the lungs inhibited murine bleomycin-induced pulmonary fibrosis (12-15). Furthermore, decorin can inhibit connective tissue growth factor (CTGF)-induced collagen production in fibroblasts (16). Taken together, these reports strongly suggest that decorin is involved in the pathogenesis of pulmonary fibrosis. The goal of the present study was to clarify the role of decorin in AE of idiopathic interstitial pneumonia (IIP).
Methods
Subjects
Fifty-six patients who were sequentially hospitalized for AE of IIP (AE-IIP) at our department between 2007 and 2014, 97 patients with clinically stable IIP (SD-IIP), and 36 healthy volunteers (HVs) were included in this analysis. Clinically stable was defined as no subjective symptoms of dyspnea or rapid deterioration on image findings for at least three months. AE was diagnosed according to a definition of AE in a previous report (17). The inclusion criteria were as follows: progression of dyspnea within the past month; new bilateral infiltrative shadows or ground glass opacities on high-resolution computed tomography (HRCT); and a decrease in partial pressure of arterial oxygen (PaO2) of at least 10 Torr or a PaO2/fraction of inspiratory oxygen (FiO2) (PF) ratio of <300 mmHg. Patients with pneumonia, heart failure, pulmonary embolism, and pneumothorax were excluded. In addition, patients whose progression of pulmonary fibrosis was clearly associated with another disease, such as connective tissue disease, hypersensitivity pneumonitis, pneumoconiosis, drug-induced lung injury, sarcoidosis, lymphangioleiomyomatosis, and pulmonary Langerhans cell histiocytosis were also excluded. The definition outlined in the 2011 ATS/ERS/JRS/ALAT joint statement was used to diagnose patients as having IPF (18), and all patients with IIP met the 2013 ATS/ERS Update of the International Multidisciplinary Classification of the IIP (19). At the time of admission, patients were diagnosed as having systemic inflammatory response syndrome (SIRS) (20) and APACHE II classifications (21) according to past diagnostic criteria. All patients had undergone steroid pulse therapy for treatment. This work was approved by the ethics committee of Fukushima Medical University (approval number: 2484), and all clinical investigations were conducted according to the principles of the Declaration of Helsinki. Informed consent was not obtained because the data were analyzed anonymously.
Measurement of serum decorin levels
First, serum decorin levels were compared among the AE-IIP patients, SD-IIP patients, and HVs. Next, serum decorin levels during AE were compared in the same patients with those in the clinically stable phase. Serum samples to evaluate decorin levels during AE were collected before starting therapies. Decorin levels were measured by ELISA (DuoSet, R&D, Minneapolis, MN, USA), according to the manufacturer’s protocol as described previously (22).
The relationship between serum decorin levels and clinical parameters
The relationship between serum decorin levels of patients with AE-IIP, and clinical characteristics such as blood laboratory findings was also examined.
The relationship between serum decorin levels and prognosis
The clinical data of a survival group and a non-survival group, as defined at 60 days after admission for AE were compared, and the Kaplan-Meier analysis was used to examine the relationship between serum decorin levels and prognosis.
Statistical analysis
Data are expressed as mean ± standard errors of the mean (SEM), unless otherwise stated. The Mann-Whitney U test or Fisher’s exact test was used to compare two unpaired groups, and ANOVA with the Tukey HSD for multiple groups. Serum decorin levels in each patient during AE and in the clinically stable phases were analyzed with Wilcoxon’s signed-rank test. We used Spearman’s correlation coefficient to analyze correlations between serum decorin levels and clinical parameters. Survival curves were made using Kaplan-Meier method, and the log-rank test was used to analyze survival rates. Statistically significance was defined as P<0.05.
Results
Clinical characteristics
Fifty-six patients with AE-IIP were hospitalized during the study period; 21 were IPF patients and 35 were IIP other than IPF (non-IPF) patients. Their mean age of 70±1 years, and 47 men and nine women were included. The patients with AE-IIP had a reduced PF ratio as well as elevated Krebs von den Lungen-6 (KL-6), surfactant protein (SP)-A, and SP-D levels (Table 1). There was no difference in clinical characteristics between the IPF and non-IPF patients, except for a predominance of males among the IPF patients. When the AE-IIP and SD-IIP patients were compared, a significantly higher white blood cell (WBC) count, lactate dehydrogenase (LDH), C-reactive protein levels, erythrocyte sedimentation rate, KL-6, SP-A, SP-D, and brain natriuretic peptide (BNP) in the AE-IIP patients, as well as a significantly lower % vital capacity (VC) and PF ratio were found (Table 1).
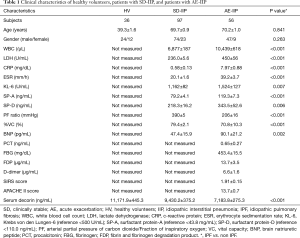
Full table
Serum decorin levels in AE-IIP patients
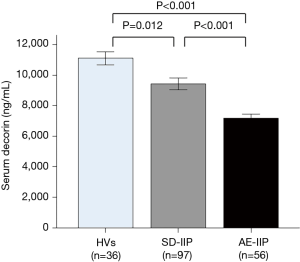
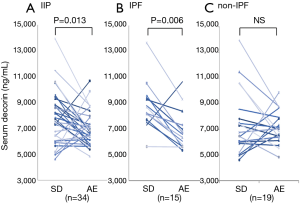
When serum decorin levels of the SD-IIP patients, AE-IIP patients, and HVs were compared, serum decorin levels in the AE-IIP patients were significantly lower than those in the SD-IIP patients and HVs (7,183.8±275.3 vs. 9,430.2±375.2 vs. 11,171.9±445.3 ng/mL; Figure 1). In addition, serum decorin levels in the SD-IIP patients were significantly lower than those in the HVs. Among the 56 patients with AE-IIP, it was possible to compare the serum decorin levels between the clinically stable phase and AE in the 34 patients. The analysis revealed that serum decorin levels were significantly lower during AE than in the clinically stable phase in the IIP (7,022.8±234.4 vs. 8,070.5±390.2 ng/mL, P=0.013 for AE and clinical stable phase, respectively; Figure 2A) and the IPF patients (6,894.5±391.4 vs. 8,778.5±458.4 ng/mL, P=0.006 for AE and clinical stable phase, respectively; Figure 2B). However, in the non-IPF patients, serum decorin levels did not differ between the clinically stable phase and AE (7,124.0±291.3 vs. 7,511.6±575.4 ng/mL, P=0.469 for AE and clinical stable phase, respectively; Figure 2C). The mean period from blood-sampling day in stable phase to hospitalization day due to AE was 556±104 days.
The relationship between serum decorin levels and clinical parameters
The relationship between the serum decorin levels of the AE-IIP patients upon admission for AE and their clinical parameters, including blood laboratory results, SIRS score, and APACHE II score was also examined. The levels of decorin in the AE-IIP patients did not correlate with any clinical parameters (Table 2). Furthermore, when the IPF and non-IPF patients were analyzed separately, no correlation was found between serum decorin levels and clinical parameters except for PF ratio in IPF patients.
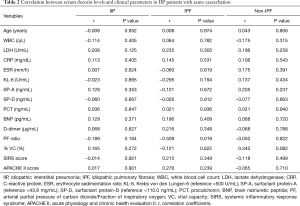
Full table
The relationship between serum decorin levels and prognosis
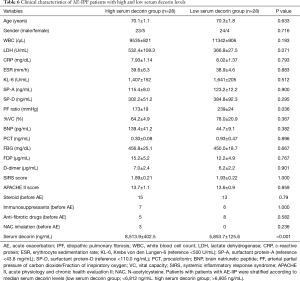
The survival rate was 53.6% (30 out of 56 patients), when prognoses 60 days after admission for AE were compared in all 56 AE-IIP patients. When the clinical characteristics were compared between the survivors and non-survivors, we observed higher fibrinogen degradation products and APACHE II levels, and lower %VC for non-survivors. There was no difference in the serum decorin levels upon admission for AE between the two groups. All patients with AE-IIP were treated with two cycles of steroid pulse therapy (methyl prednisolone 1.0 g/day, 3 sequential days) followed by 40–60 mg/day prednisolone. Although some other therapies were added to the steroid therapy in some patients, there was no difference in additional therapies such as immunosuppressant pulse therapy, sivelestat, recombinant thrombomodulin and direct hemoperfusion with polymyxin B-immobilizes fiber between the survivors and non-survivors (Table 3). In AE-IPF patients, the clinical characteristics, serum decorin levels upon admission for AE, and the therapies for AE did not differ between the survivors and non-survivors (Table 4).
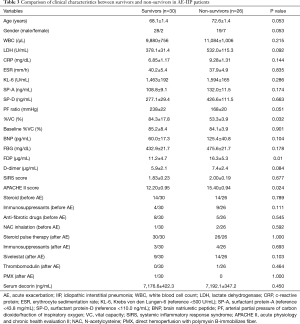
Full table
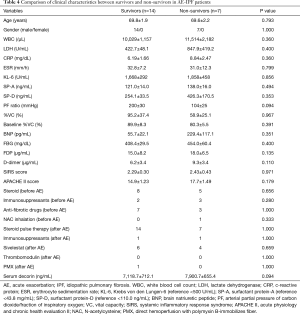
Full table
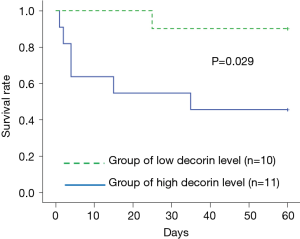
We divided the AE-IIP patients into high and low serum decorin groups, using the median serum decorin level as a reference, and analyzed the relationship between serum decorin level and prognosis. Division of the AE-IIP patients showed a significantly higher PF ratio in the low serum decorin group (Table 5); however, no correlation was found between serum decorin levels and PF ratio (Table 2). In addition, there was no difference in survival rate between the two groups. However, when the IIP patients were divided into IPF and non-IPF groups, the survival rate was significantly higher in the IPF patients with low serum decorin levels than in those with high serum decorin levels (Figure 3) despite no significant differences in clinical characteristics except for PF ratio (Table 6). In the non-IPF patients, no difference in survival rate between those with high and those with low serum decorin levels was observed, and the treatment before AE such as anti-fibrotic drugs did not differ between the low and high serum decorin groups in patients with both AE-IIP and AE-IPF.
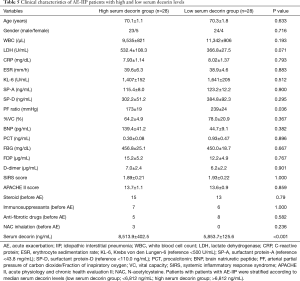
Full table
Full table
Discussion
In this study, we demonstrated that: (I) serum decorin levels were lower in the IIP patients than in the HVs, and decreased during AE; (II) serum decorin levels during AE were not correlated with any clinical characteristics except for PF ratio in IPF patients; and (III) the IPF patients with lower serum decorin levels had better prognosis than those with higher levels after the onset of AE.
Because the AE of interstitial pneumonia influences the prognosis, it is a clinically important event of interstitial pneumonia. In IPF, AE has been reported to occur at frequencies of 8.6% and 23.9% at one and three years after diagnosis, respectively, with a mortality rate of approximately 50% (2), and is the leading cause of death in Japan (23). The median length of survival after AE is three to four months in IPF patients (24,25). Although the exact mechanisms of AE have not yet been clarified, growing evidence suggests the possible involvement of alveolar epithelial injury, autoimmunity, infection and microaspiration (26). Several risk factors for the development of AE have been reported, of which a low forced vital capacity (FVC) is considered to be the most consistent (2,24,25). In addition, a recent decline in FVC (2,27,28), increased dyspnea (2,24) and previous episodes of AE (28) are reported to be risk factors. Blood markers such as KL-6, circulating fibrocytes, anti-heat shock protein 70 autoantibodies, hyaluronan and syndecan-4 have been reported as candidate prognostic biomarkers (17,25,29-32); however, no biomarker has been definitively established to date.
Decorin is a prototype of small leucine-rich repeat proteoglycans which has 12 leucine-rich repeats within a core protein and a single glycosaminoglycan chain of chondroitin or dermatan sulfate (7). Decorin interacts with a variety of proteins, such as extracellular matrix proteins, cell surface receptors, cytokines and growth factors, and is reported to have important roles in collagen fibrillogenesis, inflammation, wound repair, angiogenesis and autophagy (8,9,33).
In the lungs, decorin expression has been reported and, in IPF, was found in areas of dense collagen deposition and fibroblastic foci. In the fibroproliferative phase of acute respiratory distress syndrome, myofibroblasts within the bronchial epithelium and in hyperplastic type II alveolar cells were reported to express decorin (11). Decorin binds to collagen and has critical roles in collagen fibril formation and fibrillary spacing; furthermore, decorin-deficient mice have been reported to have a phenotype with abnormal collagen fibril morphology and skin fragility (8,34). In addition, it has been shown that decorin inhibits TGF-β activity and CTGF-induced collagen production in fibroblasts by binding to growth factors via its core protein (10,16). These results strongly suggest the important role of decorin in pulmonary fibrosis. In fact, intratracheal instillation of recombinant decorin and decorin overexpression in the lungs has been reported to attenuate murine bleomycin-induced pulmonary fibrosis (12-15).
In this study, serum decorin levels were significantly lower in IIP patients in the clinically stable phase compared to HVs. Furthermore, serum decorin levels during AE were lower compared to those in the clinically stable phase and the HVs. These results suggest the involvement of decorin in the pathogenesis of IIP in both the clinically stable phase and upon AE. However, regarding survival, IPF patients with low serum decorin levels had a significantly higher survival rate compared to those with high serum decorin levels. This result does not seem to be consistent with the anti-fibrotic effect of decorin. However, decorin is also known to be one of the danger-associated molecular patterns (DAMPs), and binds to TLR-2 and -4, leading to trigger proinflammatory signaling (30,35). It was demonstrated that stimulation of decorin induced TNF-α and IL-12 production from macrophages. In addition, it was reported that decorin levels were increased in septic lungs and decorin-deficient mice were resistant to septic inflammation (35). Furthermore, attenuation of leukocyte recruitment and reduced expression of TNF-α in the inflamed ears were observed in decorin-deficient mice (36). These results indicate the proinflammatory effect of decorin. If this is the case in AE of IPF, lower decorin levels lead to less inflammation which is consistent with better prognosis in IPF patients with lower serum decorin levels. On the other hand, in the present study, there was no relationship in prognosis between the higher and lower serum decorin groups in total IIP patients as well as non-IPF patients. We were unable to identify the exact reason of the result; however, this may reflect the difference in the pathogenesis of AE between IPF and non-IPF. In addition, although diffuse alveolar damage is considered to be a histological characteristic of IPF and non-IPF AE, other types of histological patterns, such as organizing pneumonia and extensive fibroblastic foci, have been reported (4). Furthermore, it has been reported that prognosis after the onset of AE was better in patients with non-IPF compared to those with IPF (4,6,26).
The present study has some limitations. First, this is a retrospective study of IIP patients who had developed AE, and the number of patients was limited. We found that the survival rate after the onset of AE in IPF patients with lower serum decorin levels was significantly higher when the IIP patients were divided into IPF and non-IPF patients. As the number of IPF patients who had developed AE was small and there were large variations in duration between initial stable analysis and AE, and some clinical parameters such as LDH and BNP, it is necessary to confirm our results through further prospective study of a larger population. Second, we were unable to identify the role of decorin in the pathogenesis of AE-IIP from the present study. Although we suspect the role of decorin as a DAMP and found a significant negative correlation between serum decorin level and PF ratio in IPF patients, there was no relationship between serum decorin level and inflammatory parameters such as SIRS and APACHE II scores. Because decorin has a variety of biological properties and is known to play critical roles on collagen fibrillogenesis, inflammation, wound repair, angiogenesis, and autophagy (8,9,33), the exact role of decorin in vivo might be too complex to be understood easily. In addition, we could not find the difference in prognosis between the low and high PF ration groups in IPF patients, when IPF patients were divided into the two groups according to median PF ration. However, we could not draw a conclusion whether the higher PF ration is driving survival rather than serum decorin level from the present study. Further study is necessary to clarify the exact role of decorin in the pathogenesis of AE-IIP. Finally, the exact reason(s) for the decreased levels of serum decorin of the IIP patients in the clinically stable phase as well as during AE was unclear. Increased levels of TGF-β, which is reported to inhibit decorin gene expression in fibroblasts, might be one reason (37). Moreover, the mRNA levels of decorin have been reported to be decreased in rats’ lungs after intratracheal bleomycin instillation (38). In addition, degradation of decorin by proteinases might be related to a decrease in serum decorin levels because some proteinases, such as metalloproteinases, granzyme B and cathepsin-S, which were increased in serum and the lungs of patients with IPF (39-42), have been reported to cleave decorin. Kehlet et al. recently demonstrated that an increase in cathepsin-S degraded decorin levels in the serum of patients with IPF (39). On the other hand, an increase in serum decorin levels has been reported in patients with COPD and sepsis, where protease levels are increased (35,43). It is necessary to clarify the mechanisms by which serum decorin levels are changed in patients with several pulmonary diseases in future studies.
Collectively, however, the results of this study show that serum decorin level is a potential prognostic biomarker after the onset of AE in patients with IPF.
Acknowledgements
This study was partly supported by a grant to the Diffuse Lung Diseases Research Group from the Ministry of Health, Labour and Welfare, Japan.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This work was approved by the ethics committee of Fukushima Medical University (approval number: 2484), and all clinical investigations were conducted according to the principles of the Declaration of Helsinki. Informed consent was not obtained because the data were analyzed anonymously.
References
- Kondoh Y, Taniguchi H, Kawabata Y, et al. Acute exacerbation in idiopathic pulmonary fibrosis. Analysis of clinical and pathologic findings in three cases. Chest 1993;103:1808-12. [Crossref] [PubMed]
- Kondoh Y, Taniguchi H, Katsuta T, et al. Risk factors of acute exacerbation of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 2010;27:103-10. [PubMed]
- Collard HR, Ryerson CJ, Corte TJ, et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J Respir Crit Care Med 2016;194:265-75. [Crossref] [PubMed]
- Churg A, Muller NL, Silva CI, et al. Acute exacerbation (acute lung injury of unknown cause) in UIP and other forms of fibrotic interstitial pneumonias. Am J Surg Pathol 2007;31:277-84. [Crossref] [PubMed]
- Suda T, Kaida Y, Nakamura Y, et al. Acute exacerbation of interstitial pneumonia associated with collagen vascular diseases. Respir Med 2009;103:846-53. [Crossref] [PubMed]
- Park IN, Kim DS, Shim TS, et al. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest 2007;132:214-20. [Crossref] [PubMed]
- Weber IT, Harrison RW, Iozzo RV. Model structure of decorin and implications for collagen fibrillogenesis. J Biol Chem 1996;271:31767-70. [Crossref] [PubMed]
- Gubbiotti MA, Vallet SD, Ricard-Blum S, et al. Decorin interacting network: A comprehensive analysis of decorin-binding partners and their versatile functions. Matrix Biol 2016;55:7-21. [Crossref] [PubMed]
- Neill T, Schaefer L, Iozzo RV. Decorin: a guardian from the matrix. Am J Pathol 2012;181:380-7. [Crossref] [PubMed]
- Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature 1990;346:281-4. [Crossref] [PubMed]
- Bensadoun ES, Burke AK, Hogg JC, et al. Proteoglycan deposition in pulmonary fibrosis. Am J Respir Crit Care Med 1996;154:1819-28. [Crossref] [PubMed]
- Giri SN, Hyde DM, Braun RK, et al. Antifibrotic effect of decorin in a bleomycin hamster model of lung fibrosis. Biochem Pharmacol 1997;54:1205-16. [Crossref] [PubMed]
- Kolb M, Margetts PJ, Galt T, et al. Transient transgene expression of decorin in the lung reduces the fibrotic response to bleomycin. Am J Respir Crit Care Med 2001;163:770-7. [Crossref] [PubMed]
- Kolb M, Margetts PJ, Sime PJ, et al. Proteoglycans decorin and biglycan differentially modulate TGF-beta-mediated fibrotic responses in the lung. Am J Physiol Lung Cell Mol Physiol 2001;280:L1327-34. [Crossref] [PubMed]
- Shimizukawa M, Ebina M, Narumi K, et al. Intratracheal gene transfer of decorin reduces subpleural fibroproliferation induced by bleomycin. Am J Physiol Lung Cell Mol Physiol 2003;284:L526-32. [Crossref] [PubMed]
- Vial C, Gutierrez J, Santander C, et al. Decorin interacts with connective tissue growth factor (CTGF)/CCN2 by LRR12 inhibiting its biological activity. J Biol Chem 2011;286:24242-52. [Crossref] [PubMed]
- Inokoshi Y, Tanino Y, Wang X, et al. Clinical significance of serum hyaluronan in chronic fibrotic interstitial pneumonia. Respirology 2013;18:1236-43. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644-55. [Crossref] [PubMed]
- Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818-29. [Crossref] [PubMed]
- Nikaido T, Tanino Y, Wang X, et al. Serum Syndecan-4 as a Possible Biomarker in Patients With Acute Pneumonia. J Infect Dis 2015;212:1500-8. [Crossref] [PubMed]
- Natsuizaka M, Chiba H, Kuronuma K, et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med 2014;190:773-9. [Crossref] [PubMed]
- Collard HR, Yow E, Richeldi L, et al. Suspected acute exacerbation of idiopathic pulmonary fibrosis as an outcome measure in clinical trials. Respir Res 2013;14:73. [Crossref] [PubMed]
- Song JW, Hong SB, Lim CM, et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J 2011;37:356-63. [Crossref] [PubMed]
- Leuschner G, Behr J. Acute Exacerbation in Interstitial Lung Disease. Front Med (Lausanne) 2017;4:176. [Crossref] [PubMed]
- Kondoh Y, Taniguchi H, Ebina M, et al. Risk factors for acute exacerbation of idiopathic pulmonary fibrosis--Extended analysis of pirfenidone trial in Japan. Respir Investig 2015;53:271-8. [Crossref] [PubMed]
- Reichmann WM, Yu YF, Macaulay D, et al. Change in forced vital capacity and associated subsequent outcomes in patients with newly diagnosed idiopathic pulmonary fibrosis. BMC Pulm Med 2015;15:167. [Crossref] [PubMed]
- Kahloon RA, Xue J, Bhargava A, et al. Patients with idiopathic pulmonary fibrosis with antibodies to heat shock protein 70 have poor prognoses. Am J Respir Crit Care Med 2013;187:768-75. [Crossref] [PubMed]
- Moeller A, Gilpin SE, Ask K, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2009;179:588-94. [Crossref] [PubMed]
- Sato Y, Tanino Y, Wang X, et al. Baseline serum syndecan-4 predicts prognosis after the onset of acute exacerbation of idiopathic interstitial pneumonia. PLoS One 2017;12. [Crossref] [PubMed]
- Simon-Blancal V, Freynet O, Nunes H, et al. Acute exacerbation of idiopathic pulmonary fibrosis: outcome and prognostic factors. Respiration 2012;83:28-35. [Crossref] [PubMed]
- Jarvelainen H, Sainio A, Wight TN. Pivotal role for decorin in angiogenesis. Matrix Biol 2015;43:15-26. [Crossref] [PubMed]
- Danielson KG, Baribault H, Holmes DF, et al. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol 1997;136:729-43. [Crossref] [PubMed]
- Merline R, Moreth K, Beckmann J, et al. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and MicroRNA-21. Sci Signal 2011;4:ra75. [Crossref] [PubMed]
- Seidler DG, Mohamed NA, Bocian C, et al. The role for decorin in delayed-type hypersensitivity. J Immunol 2011;187:6108-19. [Crossref] [PubMed]
- Mauviel A, Santra M, Chen YQ, et al. Transcriptional regulation of decorin gene expression. Induction by quiescence and repression by tumor necrosis factor-alpha. J Biol Chem 1995;270:11692-700. [Crossref] [PubMed]
- Westergren-Thorsson G, Hernnas J, Sarnstrand B, et al. Altered expression of small proteoglycans, collagen, and transforming growth factor-beta 1 in developing bleomycin-induced pulmonary fibrosis in rats. J Clin invest 1993;92:632-7. [Crossref] [PubMed]
- Kehlet SN, Bager CL, Willumsen N, et al. Cathepsin-S degraded decorin are elevated in fibrotic lung disorders - development and biological validation of a new serum biomarker. BMC Pulm Med 2017;17:110. [Crossref] [PubMed]
- Maher TM, Oballa E, Simpson JK, et al. An epithelial biomarker signature for idiopathic pulmonary fibrosis: an analysis from the multicentre PROFILE cohort study. Lancet Respir Med 2017;5:946-955. [Crossref] [PubMed]
- Boivin WA, Shackleford M, Vanden Hoek A, et al. Granzyme B cleaves decorin, biglycan and soluble betaglycan, releasing active transforming growth factor-β1. PLoS One 2012;7. [Crossref] [PubMed]
- Rosas IO, Richards TJ, Konishi K, et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med 2008;5. [Crossref] [PubMed]
- Keene JD, Jacobson S, Kechris K, et al. Biomarkers Predictive of Exacerbations in the SPIROMICS and COPDGene Cohorts. Am J Respir Crit Care Med 2017;195:473-481. [Crossref] [PubMed]


